William K. Oh, MD, presented “Germline and Somatic Mutations: Can They Change Therapy?” during the 24th Annual Southwest Prostate Cancer Symposium on April 13, 2019 in Scottsdale, Arizona.
How to cite: Oh, William K. “Germline and Somatic Mutations: Can They Change Therapy?” April 13, 2019. Accessed Jul 2025. https://grandroundsinurology.com/germline-and-somatic-mutations-can-they-change-therapy/
Germline and Somatic Mutations: Can They Change Therapy? – Summary:
William K. Oh, MD, discusses emerging research related to how germline and somatic mutations affect the development and treatment of prostate cancer. He emphasizes the potential for selection of targeted therapies based on genetic testing results.
Abstract:
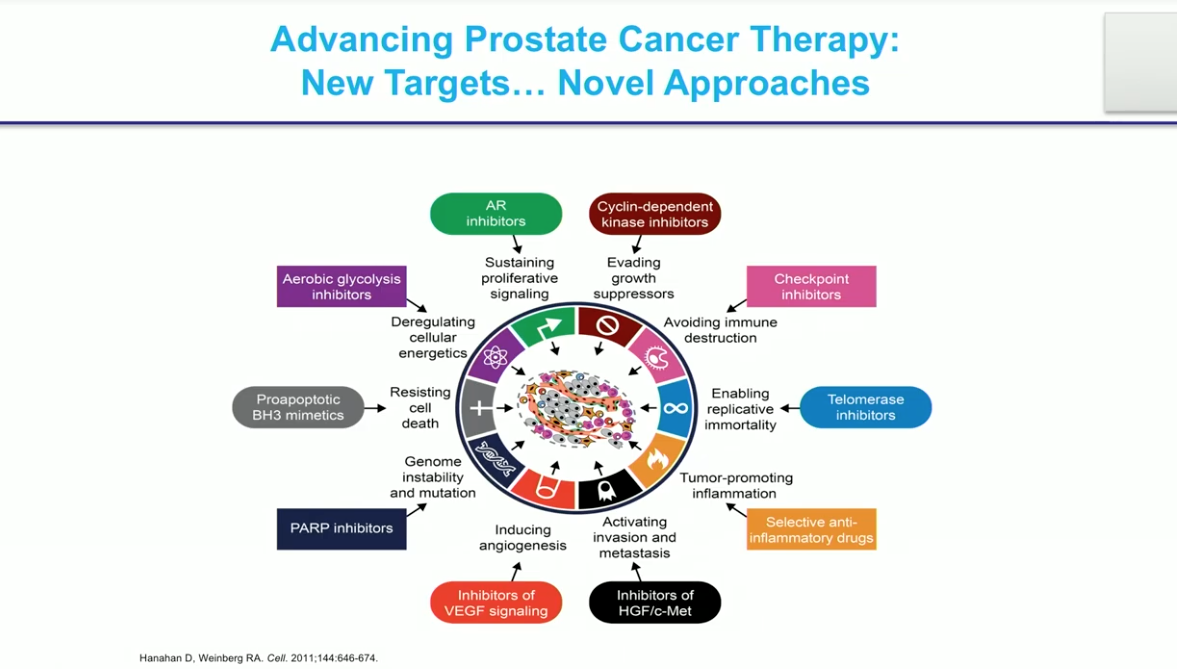
Although prostate cancer (PCa) tumors anatomically start in the same place, not all cases of PCa are the same disease. Physicians commonly differentiate clinical entities of PCa based on Gleason score, localized versus metastatic status, and castration-sensitive versus castration-resistant groupings. However, evaluating germline and somatic mutations in prostate cancer tumors may change this mentality. Looking at prostate cancer tumors on a molecular level instead of at a patient’s histology may help group prostate cancer cases in a unique way and improve drug selection. The goal of analyzing genetic mutations and differences between PCa cases is to develop and select therapies that will be more effective in each subdivision of prostate cancer.
Learning from TCGA
The Cancer Genome Atlas (TCGA) found that PCa is not a highly mutated disease. For example, the incidence of microsatellite instability is only 3%. By comparison, in colon cancer, this number in the 20-30% range. The largest rate of alterations in prostate cancer is in BRCA1 and the loss of PTEN.
A 2015 study investigated whole exome and transcriptome sequencing of prostate cancer biopsies and genetic lesions to identify genetic changes in mCRPC. The study found two main genetic abnormalities of note: genetic alterations in the androgen receptor (AR), and p53.
Androgen signaling is still important, even when patients become hormone refractory. This finding led to anti-androgens such as enzalutamide and abiraterone. There are not many other known mutations available to target in prostate cancer, and unfortunately, there are no drugs currently targeting p53.
BRCA1 and 2 Mutations
The BRCA1 and 2 mutations became a prevalent topic when Angelina Jolie found out she had the BRCA1 germline mutation after her mother died from breast cancer. It is now known that BRCA1 and 2 also influence prostate cancer. BRCA2 is the most common genetic abnormality, at 23%, in lethal prostate cancer. While 1 in 5 cases of BRCA2 mutations in prostate cancer are germline mutations, the BRCA2 mutation can also be a somatic mutation (about 12% of cases).
PARP Inhibitors
A study published in the New England Journal of Medicine found that patients with mCRPC with DNA repair abnormalities in the tumor have better radiographic progression free survival (rPFS) and overall survival (OS) when treated with olaparib. Olaparib interferes with DNA repair and only works well in patients with these genetic alterations. Interestingly, olaparib is already approved in the United States and worldwide for breast cancer patients with DNA repair alterations.
Similarly, the TRITON-2 trial found that mCRPC patients with the BRCA1 or 2 mutation responded to rucaparib, whereas other patients, including those with other gene mutations like ATM and CDK12, did not respond.
Metastatic CRPC patients who are BRCA2 carriers also demonstrate a 75% PSA response rate to carboplatin, a platinum chemotherapy, as compared to 17% in noncarriers.
Another important advancement involves the prostate-specific membrane antigen (PSMA). PSMA-617 can now be linked to lutetium ([177Lu]-PSMA-617). Lutetium 177 shows particular promise as a targeted treatment for mCRPC patients with high prostate-specific membrane antigen expression. There are ongoing clinical trials in the United States looking at [177Lu]-PSMA-617. There are some bothersome side effects like dry mouth, but in general, it is well-tolerated.
Conclusions
In summary, DNA damage repair (DDR) mutations are common, highly responsive to PARP inhibitors (like olaparib, rucaparib, and niraparib), and may synergize with abiraterone even in unselected patients.
Genomic sequencing of tumors may overall elucidate rare gene targets for therapy. DDR pathways are most commonly abnormal and may respond to PARP inhibitors or platinum chemotherapy. Some rare tumors may also respond to specific targeted therapy like AKT inhibitors. Another promising targeted treatment for prostate cancer is Lu-177 PSMA.
However, the bottom line is mCRPC patients should undergo testing for somatic and germline DDR mutations. Patient DNA testing is available by sending tests to commercial labs, consumer labs, or working with a genetic counselor.
About the Southwest Prostate Cancer Symposium
The Southwest Prostate Cancer Symposium (SPCS) is a multi-day conference that seeks to educate urologists, radiation oncologists, medical oncologists, and other healthcare professionals involved in the treatment of prostate cancer. The topics focus on current technical aspects of diagnosis and treatment of localized and advanced disease, particularly regarding imaging, technology, and training in the related devices. Dr. Oh presented this lecture during the 24th SPCS in 2019. In 2020, the 25th SPCS will also offer training sessions involving imaging, scanning, and prostate cancer treatment related devices on site. Please visit this page in order to register for future SPCS meetings.
ABOUT THE AUTHOR
Dr. William K. Oh is Chief Medical Officer at the Prostate Cancer Foundation (PCF), the world’s leading philanthropic organization dedicated to the eradication of prostate cancer and is also a Clinical Professor of Medicine in the Division of Hematology and Medical Oncology at The Tisch Cancer Institute of the Icahn School of Medicine at Mount Sinai. He is an expert in the management of genitourinary malignancies, including prostate, renal, bladder, and testicular cancers.
Dr. Oh received his MD from New York University School of Medicine. He completed his internship and residency in internal medicine at Brigham and Women’s Hospital in Boston. Dr. Oh completed a fellowship in medical oncology at the Dana-Farber Cancer Institute.
For two years prior to joining PCF, Dr. Oh was the Chief Medical Officer at Sema4, a patient-centered health intelligence company. From 2009-2020, Dr. Oh was Chief of the Division of Hematology and Medical Oncology at the Mount Sinai Health System and Deputy Director of The Tisch Cancer Institute, an NCI-designated cancer center, at Mount Sinai. Dr. Oh spent 14 years at Harvard Medical School and the Dana-Farber Cancer Institute in Boston, including service as Clinical Director of GU Oncology and Associate Professor of Medicine.
Dr. Oh has authored more than 350 original articles, reviews, and book chapters on topics relating to prostate, renal, bladder, and testicular cancers. He has edited three books on prostate cancer. He has served in key invited roles for the American Society of Clinical Oncology (ASCO), the American Cancer Society (ACS), and the American Urological Association (AUA), including the Guidelines Committee for Castration-Resistant Prostate Cancer. In addition to reviewing for prominent journals such as the New England Journal of Medicine, Journal of Clinical Oncology, and Cancer, Dr. Oh was inducted into the prestigious American Society for Clinical Investigation (ASCI) and has been repeatedly selected as a Top Doctor in New York Magazine, Castle Connolly, Best Doctors, and Super Doctors.