Robert Reiter, MD, presented “The Ups and Downs of MRI Interrogation of the Gland” at the 2nd Annual International Prostate Cancer Update on January 27, 2018 in Beaver Creek, Colorado
How to cite: Reiter, Robert. “The Ups and Downs of MRI Interrogation of the Gland” January 27, 2018. Accessed Feb 2026. https://grandroundsinurology.com/The-Ups-and-Downs-of-MRI-Interrogation-of-the-Gland/
Summary:
Robert Reiter, MD, discusses the uses of MRI in prostate cancer management, especially its ability to predict biochemical recurrence (BCR). He also explains the difference between mapping and targeted biopsies, and how MRI suspicion score and tumor volume are associated with grade, stage, and recurrence of disease.
(Twitter Question) Reveal the Answer to Audience Response Question #1
Modern multiparametric MRI consists of which of the following sequences?
- A. Diffusion-weighted imaging
- B. T2 weighted imaging
- C. Perfusion weighted imaging
- D. All of the above.
Reveal the Answer to Audience Response Question #2
PI-RAD score is based on which of the following elements?
- A. Lesion size
- B. Suspicion of extracapsular extension
- C. Seminal vesicle invasion
- D. Lymph node size
Reveal the Answer to Audience Response Question #3
Case Study- A 59-year-old gentleman has a TRUS biopsy that shows two cores of Gleason 6 disease on the right. An MRI was obtained two months later, and it shows a PI-RAD 5 lesion in the right peripheral zone. What would you do?
- A. Do a targeted biopsy
- B. Recommend active surveillance
- C. Recommend immediate treatment
- D. A or C
- E. B or C
Answer Explanation:
The answer is option D, “A or C.” A PI-RAD 5 lesion in the prostate is strongly prognostic for biochemical recurrence and a Gleason 7 or higher disease. If I see a PI-RAD 5 lesion in somebody with, let’s say, a PSA density of 0.16, I might not do a repeat biopsy on that patient. I might take them immediately to surgery or radiation, but doing a repeat targeted biopsy is not the wrong thing to do.
The Ups and Downs of MRI Interrogation of the Gland – Transcript
Click on slide to expand
Disclosures
[The only] feature that really yields a PI-RAD score is lesion size. The others are the diffusion-weighted imaging, perfusion-weighted imaging, and the Tyk2 weighted image, but none of the others. So that’s important to know when really thinking about what are some of the limitations of MRI. If you use PI-RAD as really the only thing you’re looking at on an MRI, you’re going to be missing some information from an MRI that could be potentially of importance. So my disclosures, go back through those.
mpMRI: Uses
So MRI, as you know, has many, many uses, and I think the list has increased. We got into this 15 years ago because we wanted to plan our surgeries better. There was an article from Memorial Sloan Kettering suggesting that the use of MRI prior to surgery could actually improve a surgeon’s decision making in terms of nerve sparing and what not. So, we got into it about the time that the robot came about as a way to see what was going on when we could no longer really feel what was going on, which was, of course, one of the earliest critiques of robotic surgery. And then later on when Lenny Marks joined our group in 2009, he started to use this for diagnosis, particularly with ultrasound MRI fusion biopsy. A big group at UCLA is also doing in-bore MRI biopsy, predominantly done by the radiologists. As time has gone on, MRI has been increasingly recognized to be useful for management decisions, particularly active surveillance versus therapy, as Lauri alluded to this morning, and as I mentioned, the question is whether MRI is useful for either surgical guidance or particularly for planning of focal therapy, which Nelson touched upon and which I’ll expand upon a little bit more in this talk.
PI-RAD Predicts BCR Among Patients with (Any) Biopsy Gleason 3+4
So the PI-RAD score, one of the, I think, really strengths of MRI is it is not just providing anatomical information, it’s providing functional biological information, and this is a study that we just submitted for publication, but basically we took a large group of men with Gleason 3+4 and any kind of biopsy whether it was systematic, transperineal, or a targeted biopsy, and we simply asked whether the PI-RAD score of the suspicious lesion on MRI could actually predict or prognosticate for biochemical recurrence, and as you can see here, there is actually a very strong correlation between the PI-RAD score itself in all patients with Gleason 3+4 on biopsy and ultimately biochemical recurrence with PI-RAD 5 of course being associated with the highest risk of recurrence over time.
Predictors of BCR (Univariate)
This is a paper that we just published recently, again, looking at a large collection over 300 cases where we had the MRI as well as the whole mount prostatectomy specimens, and what we looked at is what are the predictors of biochemical recurrence with the focus on looking at the PI-RAD score as well as the tumor volume predicted by the MRI, even if that tumor volume may not predict the ultimate tumor volume on whole mount, whether just the MRI region of interest tumor volume could predict biochemical recurrence, and indeed by univariate analysis, tumor volume could predict recurrence, the overall PI-RAD scores I just showed could predict recurrence, as well as the usual things, pathologic stage, grade, positive surgical margins.
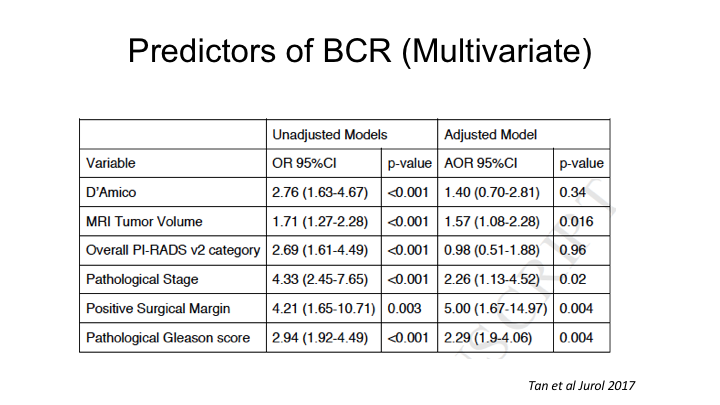
Predictors of BCR (Multivariate)
When we looked at a multivariate analysis, the one determinant of recurrence from an MRI was actually not PI-RAD score in this analysis, but MRI tumor volume, as being the strongest independent predictor of recurrence, and that is probably because PI-RADs already correlates with Gleason score, so it’s accounted for in the pathologic Gleason score to some extent.
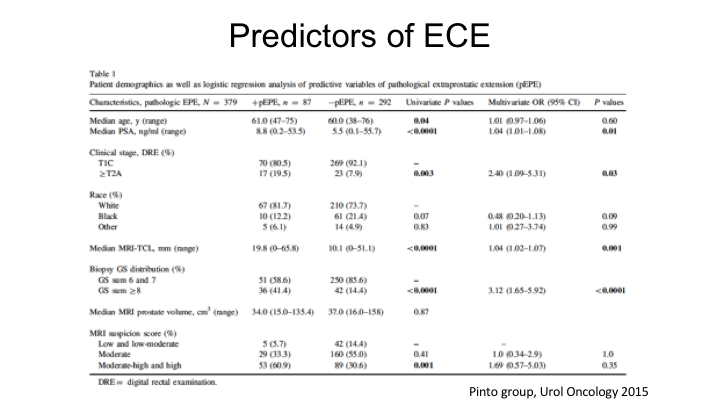
Predictors of ECE
More information you can get from MRI other than PI-RAD scores, you can actually—there are predictors of extracapsular extension that you can get, and this is work from Peter Pinto‘s group at NCIs where they looked at both the MRI suspicion score. This really predates PI-RAD, but they also looked at things such as the contact link between the tumor and the capsule, so the longer the capsular contact, the tumor contact with the capsule, the question is, “Could that predict extracapsular extension?”
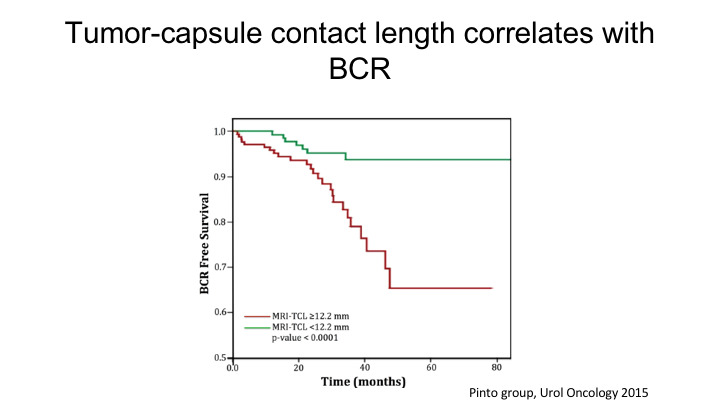
Tumor-capsule contact length correlates with BCR
And his group showed that the tumor capsule contact length was actually a strong predictor of both extracapsular extension as well as biochemical recurrence. So the point is that the MRI really does not—the PI-RAD score only accounts for diffusion, Tyk2, etc. It includes volume to some extent because the difference between PI-RAD 4 and 5 is a lesion that is less than 1.5cm or greater than 1.5cm, but it doesn’t really include any statement in the PI-RAD score about extracapsular extension or biochemical recurrence per se, and so you need to take into account the actual tumor volume on the MRI as well as tumor capsule contact length, and there will be other radiomic features that will emerge over the next coming years, which will actually improve upon the current PI-RAD score.
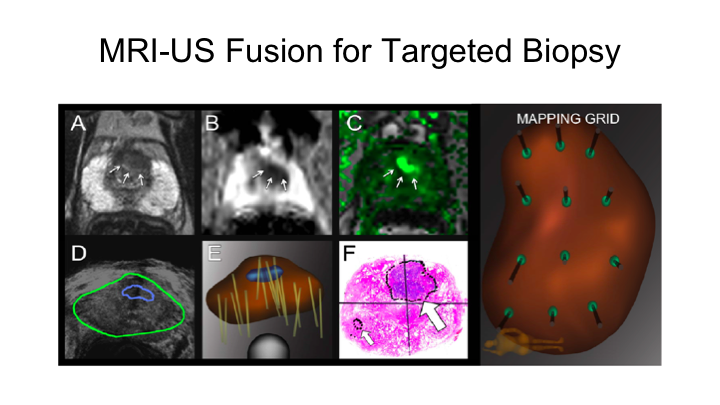
MRI-US Fusion for Targeted Biopsy
You heard a little bit about MRI ultrasound fusion for targeted biopsy. We’ve been doing this now for about nine years. There are a number of devices that can be used. You heard a little bit about this from Lauri. This is the Artemis device. We also use Urinav but there are many platforms, and those platforms can be used both for transrectal, but also for transperineal. So, you can certainly apply this MRI fusion to a transperineal platform, which is increasingly used in Europe and in places with high rates of infection after transrectal biopsy.
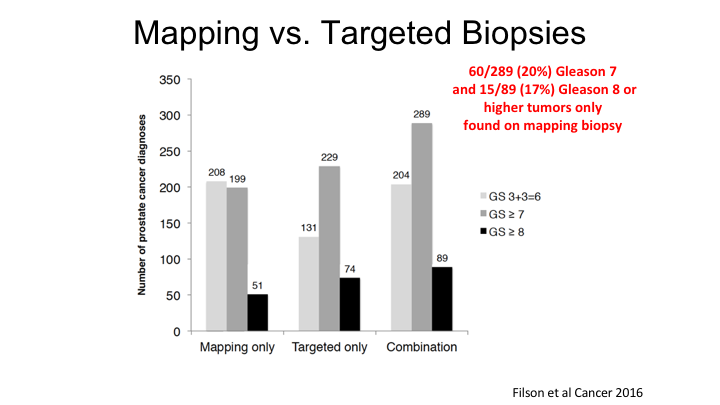
Mapping vs. Targeted Biopsies
So this is some early data that really looked at the first 1,000 transrectal ultrasound fusion biopsies that were performed at UCLA, and the key point here is that if you just look at the targeted biopsy alone, the great thing about MRI is that you reduce the detection of Gleason 6 cancers, but you maintain or even increase the risk of finding a Gleason 7 or Gleason 8 cancer. But, we continue to believe that a systematic, or mapping biopsy, basically a pre-planned systematic biopsy by the machine, can actually improve the detection of high-risk cancers, and so if you look at the total detection rate for Gleason 7, combining systematic plus the targeted, you get an additional 60 cases of Gleason 7 and 15 cases, which implies that just the targeted biopsy alone will miss about 20% of cancers, which is in line with what I’ll show you a little bit later, which is the overall detection rate of cancer—significant cancers by MRI is about 80%. So we still continue to believe that there is a role for systematic biopsy, but like anything it has advantages and disadvantage. The advantage is you are going to find all of the cancers that are probably present. The disadvantage, like a 3D biopsy we just heard about, is you are going to find all of the Gleason 6 cancers as well. So you need to take the good with the bad in terms of deciding what to do.
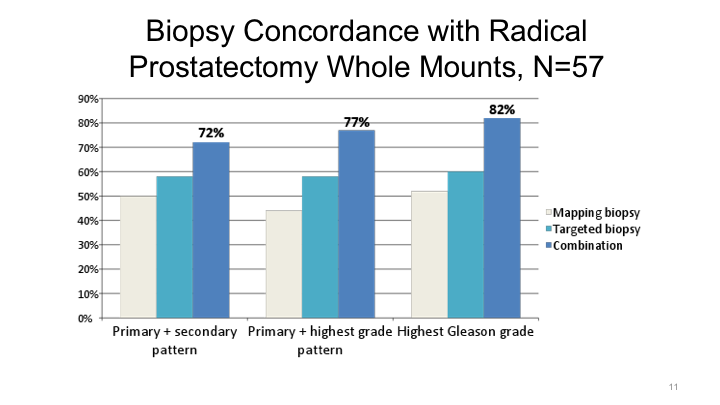
Biopsy Concordance with Radical Prostatectomy Whole Mounts, N=57
We looked at what’s the concordance rate between the targeted biopsy or a combined systematic plus targeted biopsy in the final radical prostatectomy specimen. As you know, if you just use transrectal ultrasound biopsy, you under-grade cancers about 30 to 40% of the time. If you use a targeted biopsy approach, however, you’ll only under-grade about 20% of the time, 18%. Again, the best results at least in our hands were a combination of systematic and targeted biopsy to yield the optimal score.
Results: Epstein + MRI
You heard a little bit this morning about the negative predictive value of MRI for identifying men who might be appropriate candidates for active surveillance. This is one of the papers that Lauri had on his table that we published a number of years ago looking at really the ability of MRI to aid the traditional Epstein criteria for prediction of truly low-risk cancer, and in our hands, if you combined MRI together with Epstein criteria, you really—you only missed one case of an “aggressive” cancer. So certainly the absence of visible lesion on MRI together with traditional clinical parameters of a low-risk cancer do improve the identification of tumors that are low grade and could be surveilled.
Accuracy of mpMRI for prediction of Final Pathology: Location and Volume
So how is the accuracy of multiparametric MRI for prediction of final pathology? So really, how good is MRI when you interrogate the entire gland itself by radical prostatectomy, and you compare it to what the MRI security showed? There has been a lot of controversy about this. There have been a number of studies for instance those from Mark Emberton‘s group in London that have essentially said that the sensitivity of MRI for detection of significant prostate cancer is 90, even as high as 95%, whereas others have reported like ourselves that it’s about 80%. I think part of the discrepancy has to do with number one, what we’re comparing the MRI to. We’re not comparing it to biopsy results. We’re comparing it to a radical prostatectomy. The other is what is called a positive MRI. So in Emberton’s study, which you’re probably familiar with, they got an MRI first. Then they did a transperineal template-guided biopsy, and then said if the MRI was positive, and there was a cancer, that was counted as being the positive identification by the MRI of a cancer even if that cancer was maybe not located in the same location as the MNRI suspicious lesion. So there are some technical issues with some of the papers that could account for some of the discrepancy.
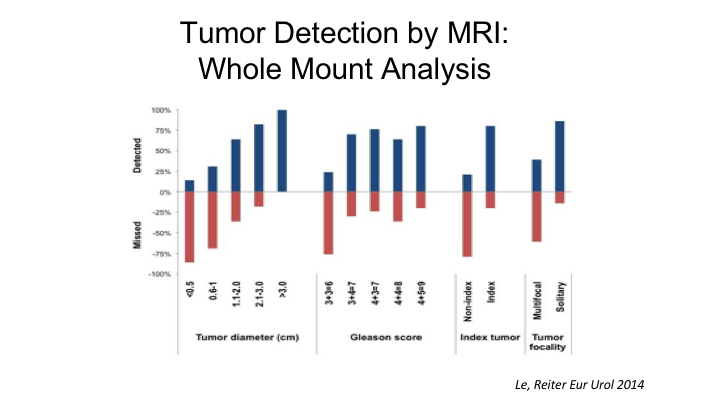
Tumor Detection by MRI: Whole Mount Analysis
But this- Nelson showed a little bit of this paper that we published a few years ago looking at 122 cases where we did an MRI prior to radical prostatectomy, and then we essentially correlated each pathological lesion with the same areas on the MRI. And what we found overall is if you look—depending on how you look at things, if you look at the index tumor, that is the largest tumor of highest grade. MRI was able to detect 80% of those, but if you look at all tumors, that is non-indexed plus indexed, MRI missed about 50% of those, so among the non-index tumors MRI missed about 80% and detected 80% of the indexed tumors. Now, part of that is owing to the ability of MRI to identify tumors of certain sizes. So if a tumor is less than half a centimeter in maximum tumor diameters, MRI really does a very poor job, only can detect about 10% of tumors that are that small and as Nelson showed, if a tumor is less than 1cm, MRI misses about 75% of those tumors. So it’s not really until you cross the threshold of one centimeter that the sensitivity of MRI for detecting cancer increases which is probably why there is a correlation between tumor volume and MRI and biochemical recurrence as well. And then importantly Gleason 6 tumors and this is probably a good thing are rarely detectable unless they are very large, whereas Gleason 7 and higher tumors are detectable about 80% of the time.
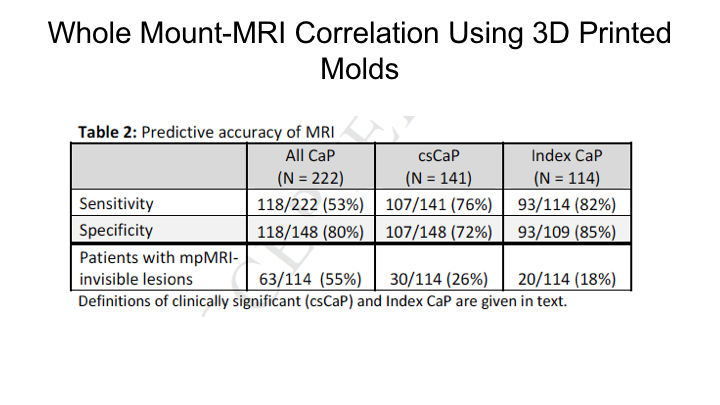
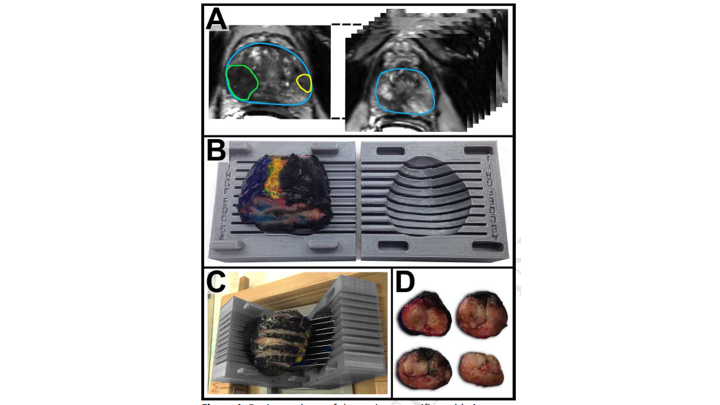
Whole Mount-MRI Correlation using 3D Printer Molds
This is a similar study that we reported just this past year where we looked at, basically, a subsequent group of patients where we actually used molds so that we could really match the MRI, the actual section of the MRI to the section of the MRI on whole mount. We found basically the same thing, we found that the sensitivity of MRI for detection of the index cancer was 80% for clinically significant cancers 76%, and for all cancers 53% so really the same thing that we had shown in the publication that Jesse Le wrote up in 2014.
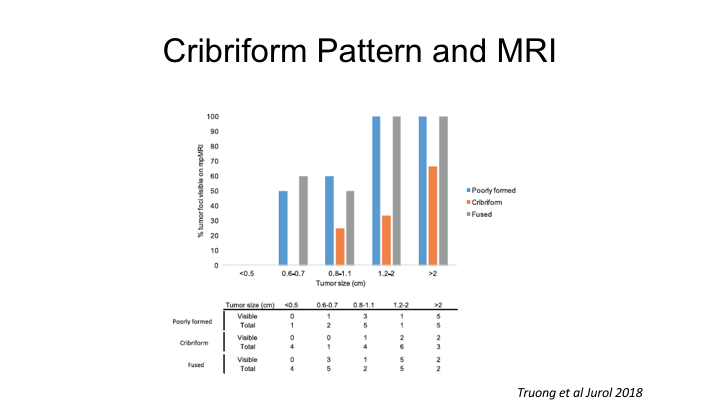
Cribriform Pattern and MRI
You heard a little bit about cribriform cancer this morning, and it’s interesting. There are clearly histologic variants of prostate cancer, which for whatever reason, not totally understood are not visible current day MRI, and this is a paper from the Rochester group showing that cribriform prostate cancers are very poorly detected with MRI, but cribriform cancers, as the Toronto group has shown are actually quite- this is actually quite aggressive biologically, so this is certainly one class that regardless of tumor size is oftentimes missed by MRI, and I think it’s important to be aware of it as a limitation.
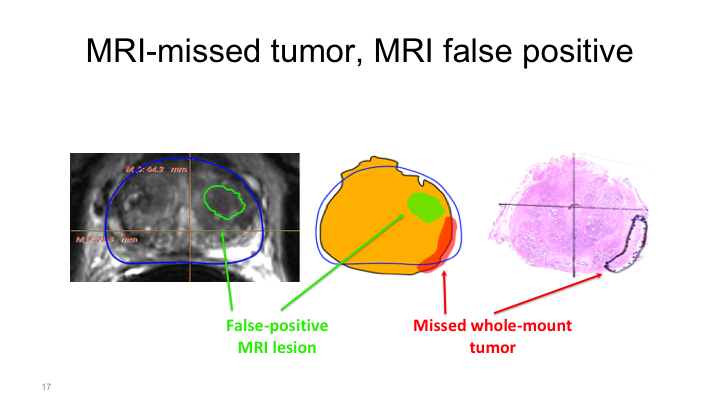
MRI missed tumor, MRI false positive
And this is what I was talking about before when I was talking about the Emberton study. In the Emberton study if there was a lesion of interest such as here, and then the patient had a positive biopsy that was called a hit basically, positive identification of a tumor with MRI. But when we do whole mount mapping studies, what we can show is sometimes you’ll see a region of interest here but the tumor is actually located elsewhere. And it may actually be true that the presence of a region of interest on the prostate, in the prostate is associated with the presence of cancer elsewhere, secondary to field effects or unknown but that needs to be elucidated further.
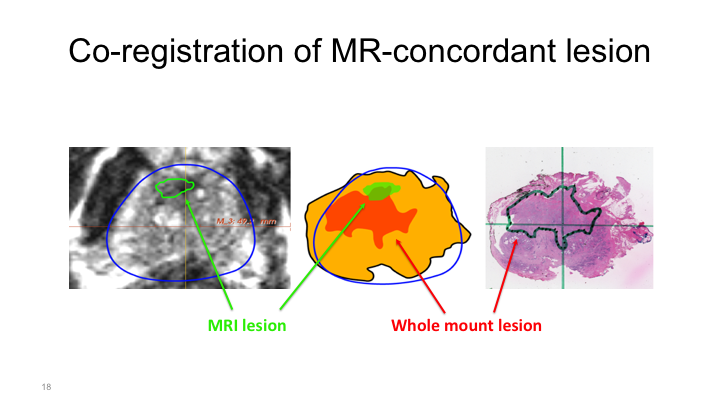
Co-registration of MR-concordant lesion
More commonly, what we see is something like this, where we have a region of interest located here, but then the whole mount shows a tumor that is obviously in this spot, so this would be considered a positive detection of a cancer, but obviously this cancer is much larger and is particular of particularly aggressive cancers, they can be pedunculated and aberrantly shaped. They’re not nice round tumors, nice circles that you can easily ablate. And so if you tried to ablate this tumor based on the MRI without this template mapping biopsy or some study such as that that we’ve heard about, you would obviously miss most of the tumor. And so this is something that years and years of working with the radiologists, the pathologists on a weekly basis at a conference I saw time and time again.
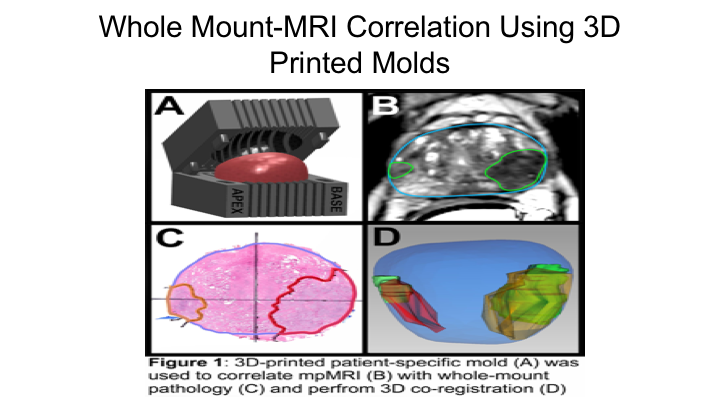
Whole Mount-MRI Correlation Using 3D Printed Molds
So, based on that, we decided to really interrogate this further, and we did this together with Lenny Marks‘ group and Alan Priester, one of our engineers who works with us, and they came up with a way to use the MRI to create a 3D mold of the prostate so that we could be sure that the sections of the prostate by whole mount were essentially in the same orientation as the sections of the MRI, and then essentially we mapped these all out measuring the tumor diameters as well as the tumor volumes and then comparing the region of interest on MRI to the final whole mount radical prostatectomy specimen.
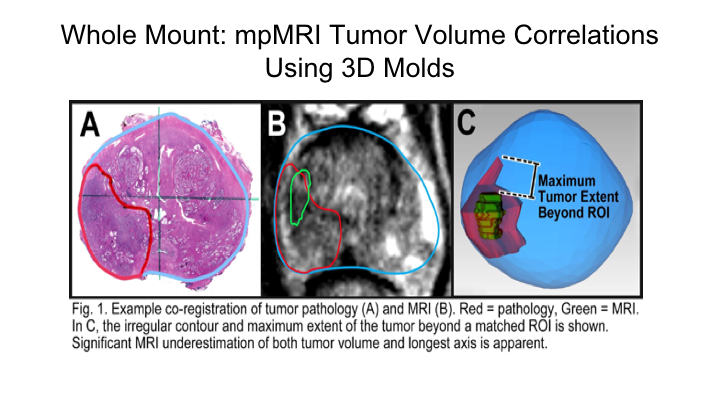
Whole Mount-mpMRI Tumor Volume Correlations Using 3D Molds
And we do this routinely really on all of our cases over the last number of years. And so what we did here, for instance, this is a tumor on whole mount, this is a region of interest that was marked by the radiologist using both T2 weighted as well as diffusion weighted imaging, and then we mapped essentially both the maximum tumor diameters as well as the tumor volumes and compared them to each other.
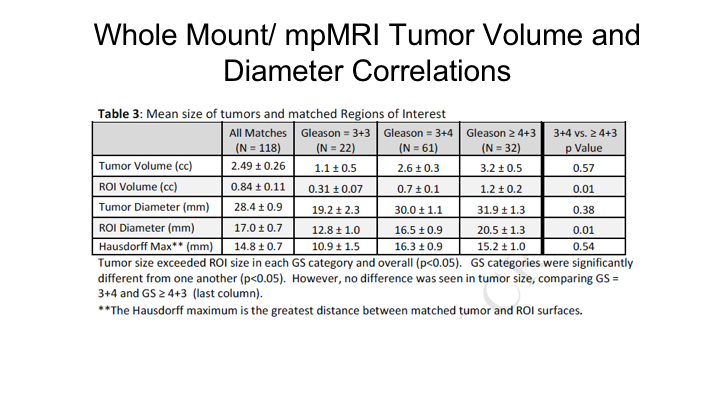
Whole Mount/mpMRI Tumor Volume and Diameter Correlations
As Nelson showed you just a little bit ago, what we found, which is what we expected, is that there is significant mismatch between tumor volume on a radical prostatectomy and the region of interest predicted tumor volume on an MRI. So if you look and importantly this increased as the Gleason score increased. So for higher-risk cancers there was actually a greater discrepancy in the predicted size of a tumor compared to the region of interest compared to actually lower-grade tumors, and this held true whether it was tumor volume or maximum tumor diameter. We measured what’s called the Hausdorff Maximum, which is essentially the greatest distance between the matched tumor and the region of interest, which averaged 15mm, so 1.5cm average discrepancy between the maximum tumor diameter on the region of interest and in the prostate.
MRI/Histology Discordance and Spatial Orientation
This shows that the mismatch occurs in multiple orientations, but particularly in the base to apex, so if you’re doing transperineal mapping, it’s particularly critical to look at basically head to toe or caudal to proximal in terms of measuring where that tumor is, perhaps even more important than measuring kind of lateral differences. But you can see that it’s very unpredictable in terms of how different it is. You have some really huge outliers, but very few fall on a curve of a perfect match.
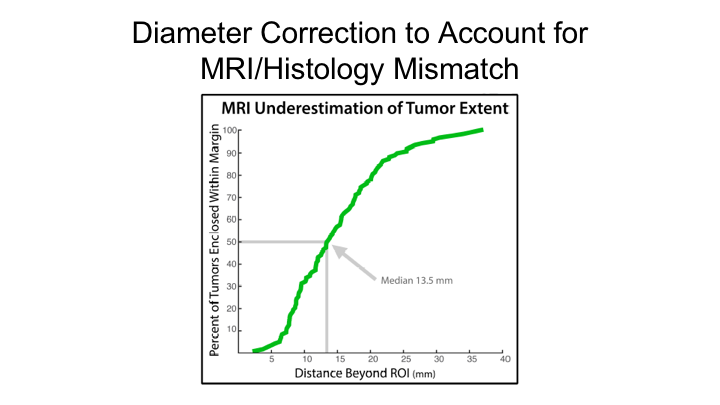
Diameter Correction to Account for MRI/Histology Mismatch
He showed this graph as well, which estimated if you were going to do focal therapy, and you were going to do what many people are doing and proposing, which is a 14mm rim or boundary around the region of interest that you would miss treating 50% of those tumors, and that to encapsulate 100% of all tumors you would have to have a 4cm margin, which of course is bigger than the prostate itself. So this just underscores that MRI alone is probably not sufficient to plan focal therapy.
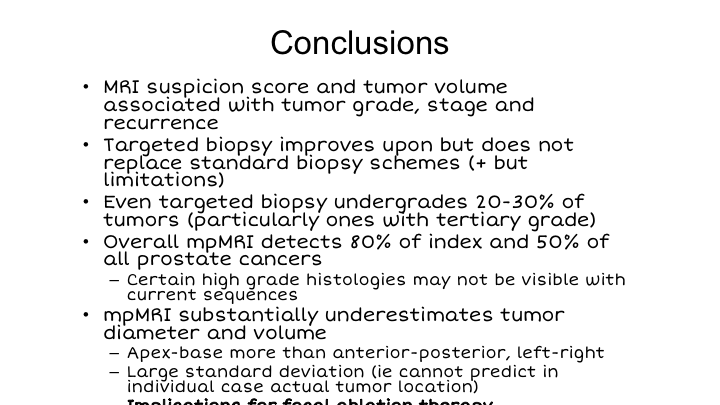
Conclusions
So in conclusion, MRI suspicion score and tumor volume are associated with tumor grade, stage, and recurrence. Targeted biopsy improves upon, but does not replace standard biopsy schemes in our opinion. Even targeted biopsy under-grades about 20 to 30% of tumors, particularly when there is a tertiary grade 5 as you can imagine. The overall detection rate for MRI of index tumors is 80% in our hands after many years, 50% for all cancers, and there are certain high-grade histologies that may not be visible with our current sequences. MRI clearly under estimates tumor diameter and volume, apex-base more than anterior-posterior. This needs to be taken into account because of its implications for focal ablation, which Fernando is now going to talk to us about in the next talk.
ABOUT THE AUTHOR
Robert E. Reiter, MD, MBA, is the Bing Professor of Urology and Molecular Biology and Director of the Prostate Cancer Treatment and Research Program at the David Geffen School of Medicine at the University of California, Los Angeles. He is currently the Principal Investigator of UCLA’s SPORE (Specialized Program in Research Excellence) program, a $12 million research grant from the National Cancer Institute to develop new diagnostic and treatment options for men with prostate cancer. Dr. Reiter’s clinical interests include robotic surgical management of prostate cancer and the use of both MRI and molecular imaging tools to manage this disease. His research is focused on the development of novel antibodies for both treatment and imaging of prostate cancer, as well as on the role of epithelial to mesenchymal transition in castration and treatment resistance. Dr. Reiter completed his undergraduate studies at Yale University and earned his medical degree at Stanford University Medical School.