Nelson N. Stone, MD, FRCSC, presented “3D Biopsy— The Complete Interrogation of the Gland” at the International Prostate Cancer Update on January 27, 2018 in Beaver Creek, Colorado
How to cite: Stone, Nelson N. “3D Biopsy— The Complete Interrogation of the Gland” January 27, 2018. Accessed. https://grandroundsinurology.com/3D-Biopsy-The-Complete-Interrogation-of-the-Gland/
Summary:
Nelson N. Stone, MD, argues that performing prostate biopsies is beneficial for detecting malignancies and lesions and cannot be replaced by MRI, despite controversies. He describes the transperineal mapping biopsy (TPMB) procedure, the inadequacies of current technology, targeted focal therapy, and future directions in these techniques.
(Twitter Question) Reveal the Answer to Audience Response Question #1
How many prostate biopsies are anticipated in the US in 2018?
- A. 1,250,000
- B. 1,000,000
- C. 750,000
- D. 500,000
Reveal the Answer to Audience Response Question #2
Finish the sentence- The false negative rate for TRUS biopsy is:
- A. 10%
- B. 20%
- C. 30%
- D. 40%
Reveal the Answer to Audience Response Question #3
mpMRI fails to identify multifocal lesions < 10 mm (all grades) in diameter in about what percent?
- A. 15%
- B. 30%
- C. 50%
- D. 75%
Reveal the Answer to Audience Response Question #4
Finish the sentence- The main reason transperineal mapping biopsy isn’t done more frequently because:
- A. It is inconvenient.
- B. It requires multiple in-line puncture because of the short specimen length.
- C. There is no mechanism to record the location of the biopsy sites.
- D. I am not experienced in transperineal procedures.
Reveal the Answer to Audience Response Question #5
Finish the sentence- A transperineal mapping biopsy performed with a 15 gauge needle with 5mm spacing can detect a lesion as small as:
- A. 0.1cc
- B. 0.25cc
- C. 0.5cc
- D. 1.0cc
3D Biopsy— The Complete Interrogation of the Gland – Transcript
Click on slide to expand
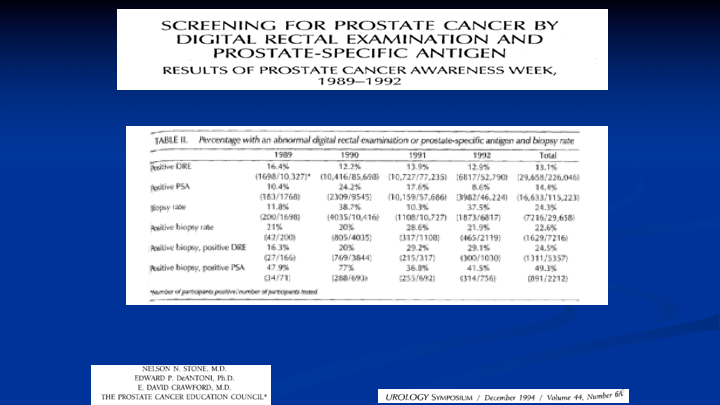
Screening for Prostate Cancer by Digital Rectal Examination and Prostate-Specific Antigen
This was the results of our first four years of the PSAW, Prostate Cancer Awareness Week, and what we found was that in terms of examining the patient, 13% came in with a positive DRE. 14.4% had an elevated PSA, and of those men who had a positive DRE, 25% were positive for prostate cancer. And of those men with a positive PSA, an elevated PSA, almost 50% had prostate cancer. Now we know the world is very different today because those numbers are not what represent the current population, but in 1989, 1990, 1991, 1992, when this data was put together, we were doing biopsies using a spring-loaded biopsy device, using a transrectal ultrasound, and just about everybody is still doing the same thing.
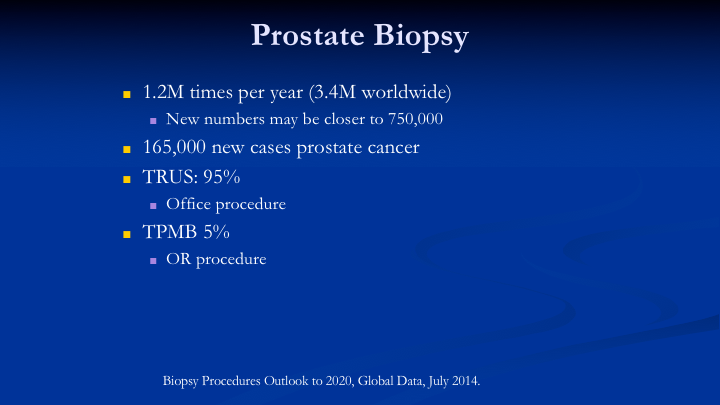
Prostate Biopsy
So it used to be that we did have 1.2 million biopsies per year, but the new numbers are about 750,000. So we’ve experienced a significant decline in the number of biopsies, and I think we all know why. It’s backlash from the Preventive Task Force. It’s the backlash I think from patients being told to come in every year to have repeat biopsies because they’re on active surveillance. There’s a lot of reasons why, and we’re trying to do things differently. We’re trying to use markers. We’re trying to use MRI. I’ll show you some of the MRI data. This year we have a decrease in the number of new cancers projected by the American Cancer Society to be 165,000. Last year it was 180,000. So that is about a 10% drop, which probably reflects a decrease in PSA testing and biopsies, but also interesting, there is an increase in the number of prostate cancer deaths this year form 24,000 to 29,000. So TRUS biopsy is done 95% of the time. It’s an office procedure, and the mapping biopsy, which has to be done typically in the OR or the AC is only done about 5% of the time. It’s done a lot more frequently in Europe and in Australia, but much less frequently in the United States.
Why Interrogate the Prostate?
So this is a term actually that David Crawford came up with about interrogating the prostate. So why would you want to do that? Why would you want to know what the pathology is as best as you can in the prostate by a biopsy? Dave actually said this to me, I don’t know, in the early 90s. If I could just know by a biopsy what’s in the prostate, we could know what to do. We still don’t know an answer to that, but maybe we can come up with a solution. So we want to determine the true pathology, really want to know what that patient’s risk of metastasis and death from prostate cancer. So today we use TRUS. We use MRI, and we put patients on surveillance, and you saw some of the data this morning, about 30%. Some people report as high as 50% of those men who go on active surveillance eventually have active treatment. So if it’s 50%, we’re not doing a very good job. If it’s 30%, we’re not really doing a good job, and it’s not always because we find worse pathology that we end up intervening. It’s also because I think it’s about half of the cases, the patients get tired of being surveilled and worried about going.
We can debate the reasons why, but the numbers are true. 30% go on to treatment regardless of why, the reasons there, and to me that’s not the best solution in terms of how we should be managing patients. I believe we’d like to know the precise location of the tumors, and I think, again think, I don’t know for sure, that down the road there is potential to make prostate cancer more like breast cancer. We don’t take off breasts in the majority of patients anymore. We treat the lesion, but there’s potential I think that more than 50% of patients who need treatment can actually keep their prostate if we had the information to tell us where the lesion or lesions. You heard from Scott Lucia this morning that the average number of lesions is two, and 70% have multifocality. So it’s the lesions—we can find the lesions and feel secure that we know where they are, and that we’re adequately treating them. Then we can leave the prostates in in most men.
Bottom Line-TRUS Bx
So bottom line TRUS is only positive in 20 to 25% of the patients. 30%–that was one of the answers need a repeat biopsy because of false negatives. I think it has limited usefulness for selecting patients for active surveillance. It really can’t be used for focal therapy because we’re missing too many lesions, but it’s hard to change what we do because we’re very comfortable what we do. And it’s going to be a big job to get people to switch over to something they’re not familiar with, and we’re going to need to prove that it actually has a benefit.
Multifocality and Prostate Cancer Detection by Multiparametric Magnetic Resonance Imaging: Correlation with Whole-mount Histopathology
Here’s some of the MRI data from—this is from UCLA, Lenny Marks’s group, and this is another one of my questions. MRI misses 73 to 89% of lesions smaller than 10 mm. This is based on radical prostatectomy whole-mount sections compared to the MRI. And even if you’re saying, okay, forget about those Gleason 6’s because they don’t show up very well on MRI, and you are focusing just on the 7’s and 8’s, its’ still missing 29 to 38% of the multifocal lesions.
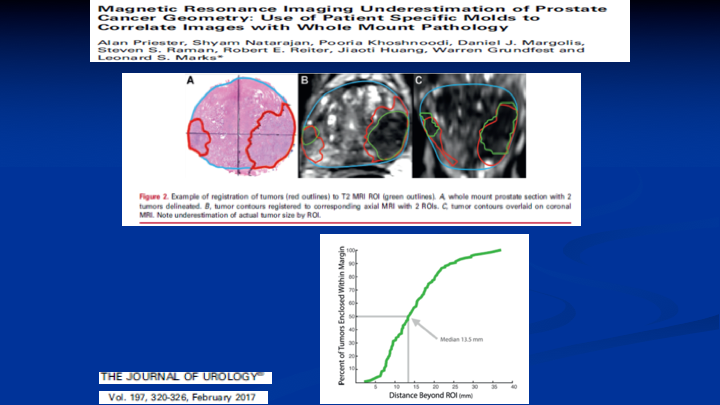
Magnetic Resonance Imaging of Underestimation of Prostate Cancer Geometry: Use of Patient Specific Molds to Correlate Images with Whole Mount Pathology
Here’s another paper out of the UCLA group where they were looking at the ability for the MRI to determine the lesion size. So on the left you see the whole mount and the prostate cancer circled in red. On the right you see the corresponding co-registration between the whole mount, and the MRI, and the lesion on your left there on that first image. That’s pretty good concordance. You could build a 1-cm margin around that lesion and say I got it, but look at the lesion on the left on both images. If you built a 1-cm margin, you’re going to be missing the boundaries of that lesion. So MRI, and this is well known now, does not define really well the borders of the lesions when you relate them to the whole mount prostatectomy specimens. So how big do you make your ablation if you’re going to do targeted focal therapy using MRI as your indicator of where the lesion is?
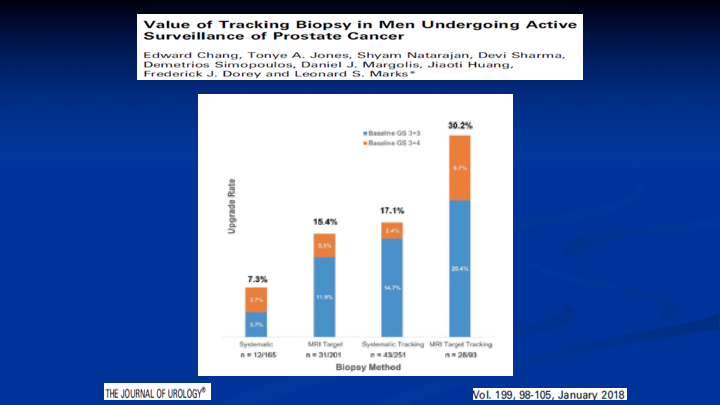
Value of Tracking Biopsy in Men Undergoing Active Surveillance of Prostate Cancer
What about active surveillance? Here’s a paper. Again, it’s from the UCLA group where they did an MRI. They selected patients for surveillance based on the MRI results, so those men with negative MRIs didn’t get a biopsy. Went on surveillance. They repeated the MRI and redid the biopsies a year later, and 30% of the patients had upgraded disease, which probably means that 30% were missed, which is the same 30% I showed you before on what does MRI miss with Gleason 7’s and above. It was 30%.
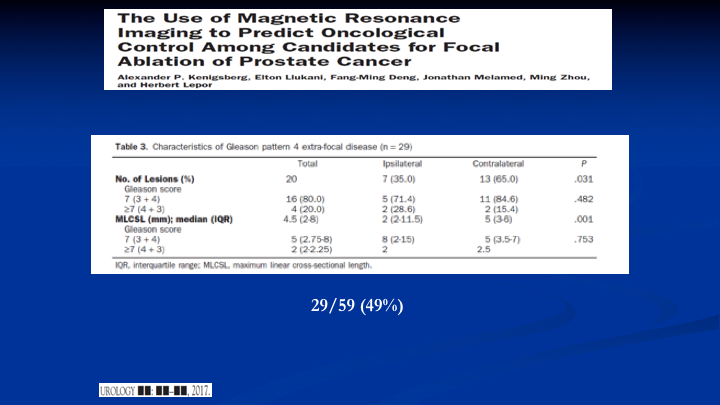
The Use of Magnetic Resonance Imaging to Predict Oncological Control Among Candidates for Focal Ablation of Prostate Cancer
And lastly, here’s a new paper out of NYU because everybody should know that Sameer Tunazia is there, and they have got some of the best MRI units in the world. They’re now using 7 tesla units. And what they did is they took patients, used the MRI to find the lesion, so the index lesion. Then they planned out a focal therapy based on the MRI and then those patients went and had radical prostatectomy. They didn’t do the focal therapy, and when they looked at the field, they would have ablated with the MRI guided focal therapy. They found, again, looking at Gleason pattern four and above, they would have missed 49% of the lesions because they were out of the planned focal therapy field. So, yes, MRI has helped us, but it’s not the be all, end all, and people will get led down the wrong pathway if they start doing things without recognizing the limitations.
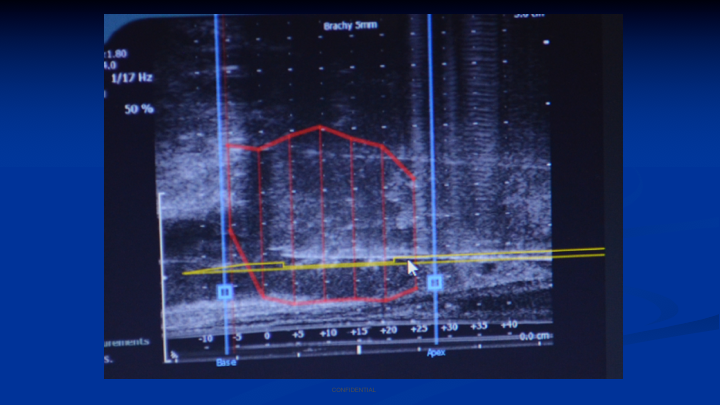
Brachytherapy Grid – Current Biopsy Technique
This is my colleague with one of my former fellows in Athens, Greece doing a transperineal mapping biopsy. You can see he’s using a brachytherapy grid. He’s putting the biopsy needle through the grid. He’s looking at the ultrasound image, and you can make out the little white reflection of the needle going through the apex, and it stops halfway through the prostate. So this is what we do today. This is what Dr. Crawford has been doing probably for 15 years in his practice. I was doing it at Mt. Sinai in my practice because I was using this to look for patients who had failed brachytherapy and they had slow-rising PSAs, and I was getting no results for transrectal biopsies. So for me moving from the transperineal brachytherapy to transperineal biopsy was an easy transition.
Inadequacy of Current Technology
So when I moved to Colorado in 2012, I got together with Dave, Scott Lucia, and other members of the group because we were like-minded in doing the transperineal biopsy. Dave had worked with the pathology predecessor to Scott Lucia, Gary Miller, I don’t know if you remember, right after—the year after we had that first prostate cancer awareness week. That was the year of your first meeting in 1990. Both of us were at and Bob Donohue, I think, the three of us. So I got together with the group, and we sat down, and we said we love the way transperineal biopsy is done, but people are not going to do it for all of the reasons you can see here. The biopsies take too short a specimen, and they deflect, so if it’s moving all over the place how are you going to know where you’re at? There’s no real good software to record the location of every site within the prostate that you want to biopsy and if you were able to take a longer biopsy, what would you do with that tissue if you needed the pathologist to look at it, and it had to be delivered to the pathologist intact, because most of the time the tissue comes in pieces.
So if you’re looking for localization information along the core, you’re not going to get it. So that was a challenge we faced, and we brainstormed back in 2012 what do we need to do to try and help solve this problem.
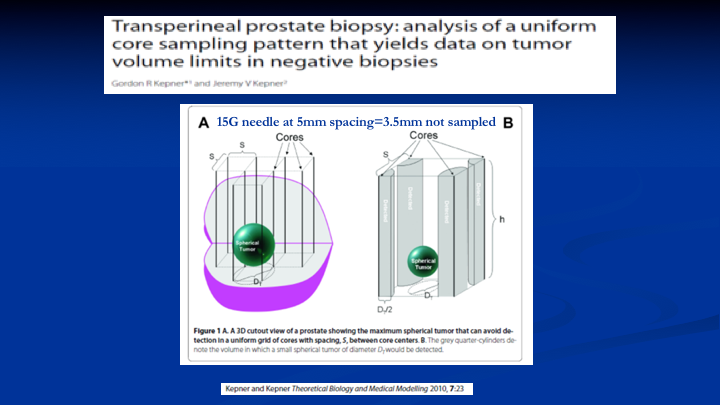
Transperineal prostate biopsy: analysis of a uniform core sampling pattern that yields data on tumor volume limits in negative biopsies
So the first thing we did is we pulled this article by Kepner and Kepner, 2010. What Kepner and Kepner said was if you could drill holes in the prostate and those holes are biopsies were a certain distance apart and they were a certain size you could define the size of the lesion that you would miss within the gland, so that math translates out into—if you took a 15G needle and did biopsies at every 5mm, the spacing that you would not sample would equal 3.5mm. That sounds pretty good because that’s pretty small. So there’s going to be a question at the end about that.
TPMB Software
The next challenge, so here we’re doing—here’s the software in its early design phase. So you see the representation of the needle is straight, and this is no different than a representation you would see with MRI fusion software, but look what’s happening to the needle. It’s bouncing above the representation. And if I want to record the exact location of the needle because I want to find a Gleason 8 prostate cancer that’s 0.3 cubic centimeters, I can’t have that needle bouncing all over the place. So we had to figure out how to fix that problem.
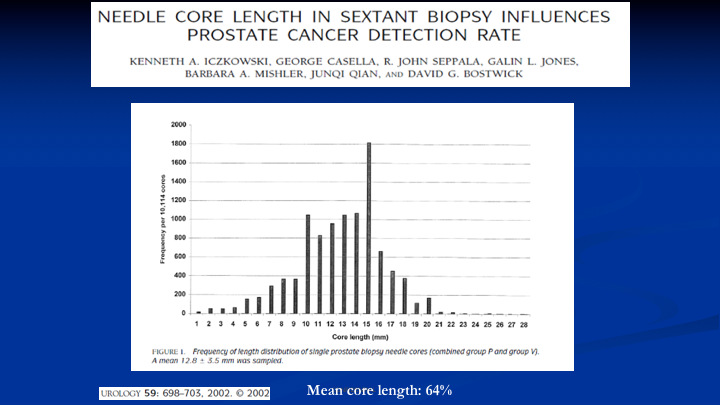
Needle Core Length in Sextant Biopsy Influences Prostate Cancer Detection Rate
The other problem we needed to fix is when you use your standard biopsy needles in the office, and it’s got a 20mm or 2cm core bed, the average length you get of a specimen is 12mm, 60%. And there’s a fair amount of data that’s here. This is one of the papers that says when the length of the core is more substantial, you increase the diagnosis of prostate cancer. But we had another reason, and that is because if we want to take a longer biopsy if we don’t get the full length of the core, we don’t know what’s in the tissue that’s missing from that core. So that was another problem to solve.
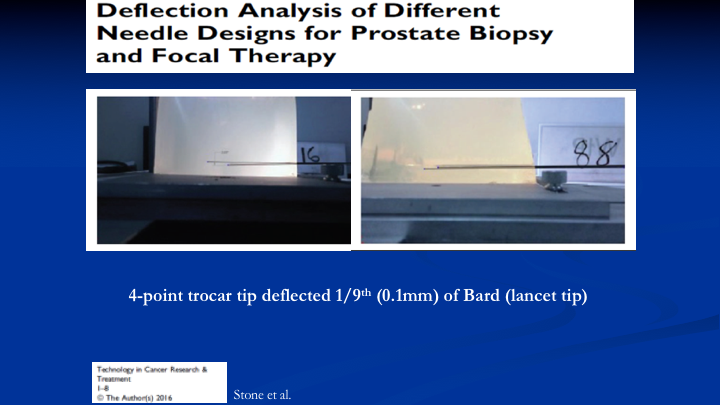
Deflection Analysis of Different Needle Designs for Prostate Biopsy and Focal Therapy
So the first problem we addressed by looking at the way the typical needle, which is lancet tip bounces around when you fire into a jelly that reflects the density of the prostates on the left. You see your Bard needle and the deflection. We eventually came up with a four-point trocar tip needle that has one-ninth the deflection, which is 0.1mm of the Bard needle. So we solved that problem.
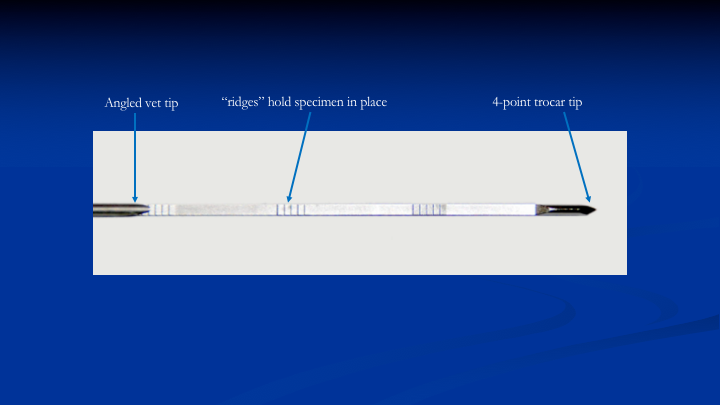
Ridges in Needle Core Bed
The next problem is how do you get that 60% to 100%. So we figured out that if we put these ridges in the needle in the core bed. As the cannula slides over the needle to cut the tissue and push it into the core bed, instead of the tissue sliding along that core bed, it’s going to be held in place. That’s exactly what ended up happening in the lab when we tested this needle between 20 and 60mm. You could get 95% of the tissue you wanted.
Technology and Engineering
So now we have a needle that can take a specimen between 20 and 60mm. So whether you’re doing a TRUS fusion biopsy and you got a lesion that’s anterior or you can put this in the rectum into the posterior capsule, dial the distance and take a biopsy that spans 5cm if that’s what you require. If you’re doing a transperineal biopsy, you can take one biopsy from apex to base regardless of where you are in the prostate as one core not do the multiple sticks.
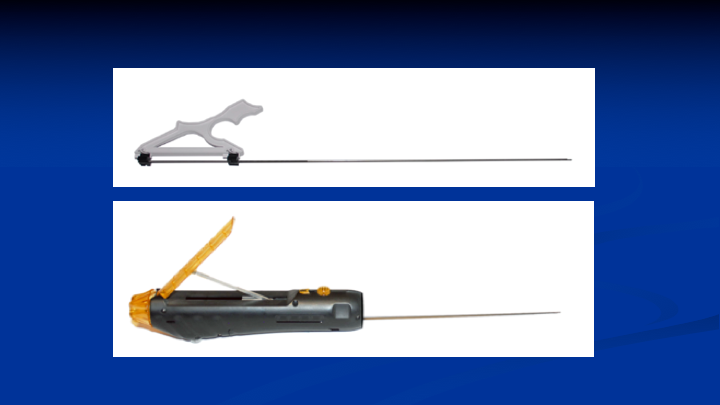
Insertion Tool and Biopsy Device
We made it simple so that’s what the needle and the insertion tool looks like, and that is what the biopsy device looks like.
Specimen handling subject to fragmentation misalignment and mishandling
And lastly say you could take a 5cm core. What are you going to do with that tissue because you’ve got to get it to the pathologists so they can look at one end, call it the apex or the anterior capsule and look at the other end and tell you where on that tissue the cancer is and how long it is. So if you’re doing what we did. This is back in Greece picking up the specimen with a needle, and dropping it into a vial of formalin, that’s what they do at the University of Colorado with the forceps there’s no way Dr. Lucia is going to tell Dr. Crawford what’s going on in that full length of tissue. It’s not happening so we had to come up with a device that would manage the longer specimens, and this is what it looks like.
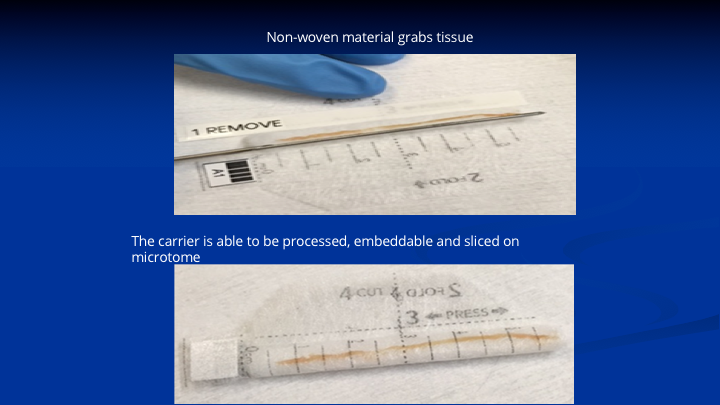
Specimen handling subject to fragmentation misalignment and mishandling Part II
This is a woven polyester fiber fabric impregnated with proprietary chemicals. That’s a 6cm specimen on a cadaver with our needle showing how it lays on the device. The lower specimen shows you close the device. That goes into formalin. It goes to pathology. It gets cut in half because remember the cassettes to process these specimens are only 3cm long. So they stain one end, that’s the base, one end, the apex, another color, they cut it in the middle. They lay the two pieces in the processing cassette. It goes through all of the steps of processing and dehydration paraffin impregnation, sectioning on the microtome staining, and being read by the pathologist. So now we have a mechanism to protect the specimen. If you’re looking along that specimen, and you’re going from one end to the other, the pathologists are going to tell you, okay the cancer starts at 2cm, it goes for 1cm and there’s no more cancer. He gives you the grade and the length of the tumor, it’s location. It gets back loaded up into the software, and then you’re ready to go.
Targeted Focal Therapy
So this is the prototype software that’s being used in a phantom. This is not used in a patient, so we had three lesions, simulated lesions that we found in this phantom of the prostate. So then the question is, just like with the MRI, how big is that lesion, because all we know is that we’ve got a biopsy that goes from base to apex, but we don’t know the diameter of the cancer outside of the lesion. So the way that we—I figured out we could determine that, is if you have all of these blue needles around it that have no cancer in them, then the theoretical diameter of the cancer could extend out to the next negative needle around it. So now that’s what I’ve done here. I’ve simulated this as a tool in the software that allows you to grow the circumference of the cancer. That information then can be exported in DICOM, and then you can marry that real time to any type of treatment planning system for doing focal therapy.
This also puts the onus on the urologist because I’m telling you 5mm, but the real life is if you’ve done brachytherapy, you know the needles never end up in the exact same place. So if you’ve got needles that are 7mm apart or 1cm apart, you’ve got the record on the post-biopsy file. You grow the lesion out to that next negative needle and that is your zone of ablation if that is what is appropriate for the patient.
Conclusions
So in conclusion, 3-D transperineal approach utilizing software, designed with a new gun and needle, has the capability of detecting lesions as small as 3.5mm. Can this platform serve as a virtual prostatectomy, interrogate the prostate? Dave, I think it does, but we need to prove it clinically. That is to come. When we decide to do targeted focal therapy, that’s going to be another issue. Do you want to target Gleason 7’s or do you want to target Gleason 8’s. What happens if you had a 0.2 cubic centimeter Gleason 8 in the prostate? Do you need to take that prostate out, or could you just abate it, and leave the prostate in? And of course we have to demonstrate proofs of concept, and these devices are not approved for clinical use.
ABOUT THE AUTHOR
Nelson N. Stone, MD, is Professor of Urology, Radiation Oncology, and Oncological Sciences at the Icahn School of Medicine at Mount Sinai and chief medical officer at Viomerse, Inc.
Dr. Stone earned his medical degree from the University of Maryland in 1979. He completed a Residency in General Surgery in 1981 at the University of Maryland, followed by a Residency in Urology at the University of Maryland. He then completed a Fellowship in Urologic Oncology at Memorial Sloan-Kettering Cancer Center and a Research Fellowship in Biochemical Endocrinology at Rockefeller University in 1986. He was Chief of Urology at Elmhurst Hospital Queens from 1986-1996.
Dr. Stone has founded several medical companies and serves on the editorial board of many scientific journals. He is a member of many professional societies, including the Prostate Conditions Education Council, the Society for Minimally Invasive Therapy, the New York State Urological Society, the American Association of Clinical Urologists, and the American Urologic Association. Dr. Stone has participated in approximately 25 research studies on prostate cancer and has authored more than 500 articles, abstracts, and book chapters, primarily on prostate cancer. He invented the real-time technique for prostate brachytherapy in 1990 and has trained more than 5,000 physicians worldwide through his company ProSeed. His most recent company, Viomerse, creates synthetic body parts (phantoms) for surgical training and has recently released an extended reality remote training platform.