Juanita M. Crook, MD, FRCPC, presented “Long-Term Outcomes of HDR” during the Southwest Prostate Cancer Symposium on April 15, 2018 in Scottsdale, Arizona.
How to cite: Crook, Juanita M. “Long-Term Outcomes of HDR.” April 15, 2018. Accessed [date today]. https://grandroundsinurology.com/long-term-outcomes-of-hdr/
Long-Term Outcomes of HDR – Summary
Juanita M. Crook, MD, FRCPC, discusses the radiobiologic rationale for high-dose-rate brachytherapy (HDR BT) and its technical advantages. She then reviews the evolution of HDR fractionation and recent toxicity reports for both HDR BT boost and HDR BT monotherapy according to prescribed dose and fractionation.
Rationale for HDR Brachytherapy
The low alpha-beta ratio for prostate cancer favors a large dose per fraction. There are several technical advantages to high dose rate prostate brachytherapy, such as dose-optimization through manipulation of the source dwell positions and durations. HDR BT also does not present practical challenges like organ motion, setup error, seed migration, or alteration of delivered dose. Furthermore, HDR BT is also economical in terms of radiation material. Clinicians can use one iridium-92 source for multiple patients and over a 90-day period.
Application of HDR Brachytherapy
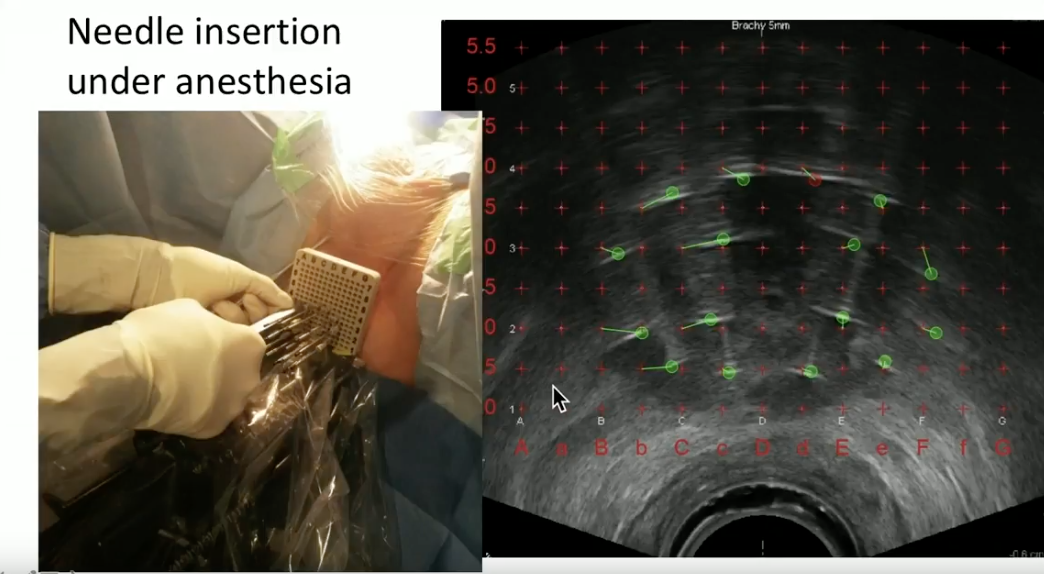
Dr. Crook illustrates her method of performing HDR BT in clinical practice. With the patient under general anesthesia, she inserts the needles through a template under live ultrasound guidance. She then explains her process of needle track identification, contouring the prostate and adjacent organs, inverse planning the dose distribution, and use of the probe to increase space between the rectal wall and radiation delivered to the prostate.
Patient Selection
Patients with large glands, who have had transurethral resection of the prostate (TURP) within the three months, or significant pubic arch interference are not good candidates for HDR BT. Additionally, patients must be able to take a lithotomy position and be fit for anesthesia. If the patient has an International Prostate Symptom Score (IPPS) score of less than 20, or obstructed voiding and retention, physicians should correct those symptoms before considering HDR BT.
Evolution of HDR Fractionation in the Past 20 Years
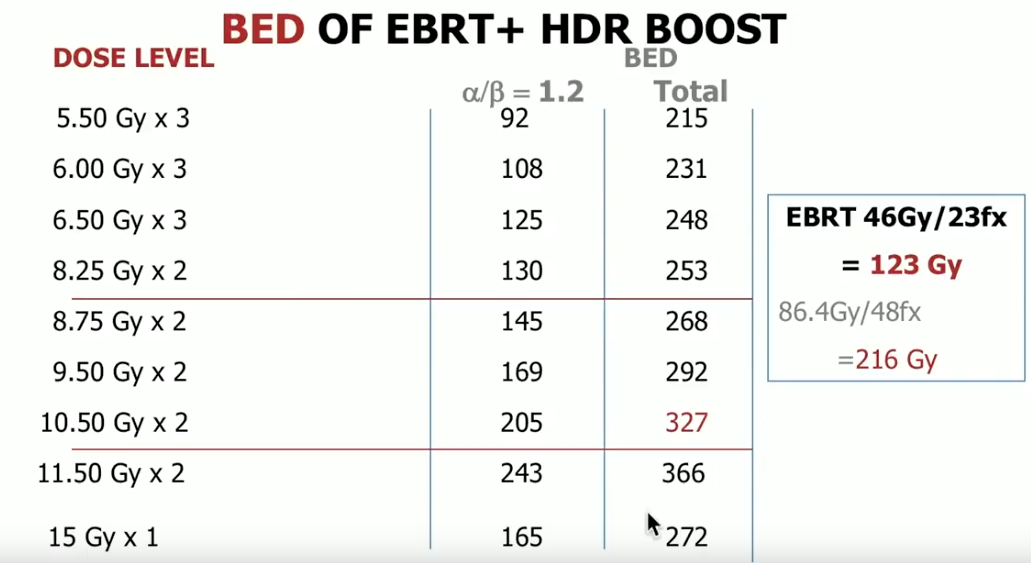
Since its emergence, the dose of low-dose-rate (LDR) brachytherapy has been 145 Gy and has not changed. Also, the dose for brachytherapy boost has either been 110 Gy or 115 GY. Therefore, there has been great uniformity in the literature surrounding this modality. Conversely, HDR BT has been in evolving since its emergence. It has changed from utilizing multiple smaller fractions to fewer smaller fractions, only serving as a boost in combination with external beam to serving as a monotherapy, and from using CT-based planning to ultrasound-based planning.
HDR Brachytherapy Results Published in the Past 18 Months
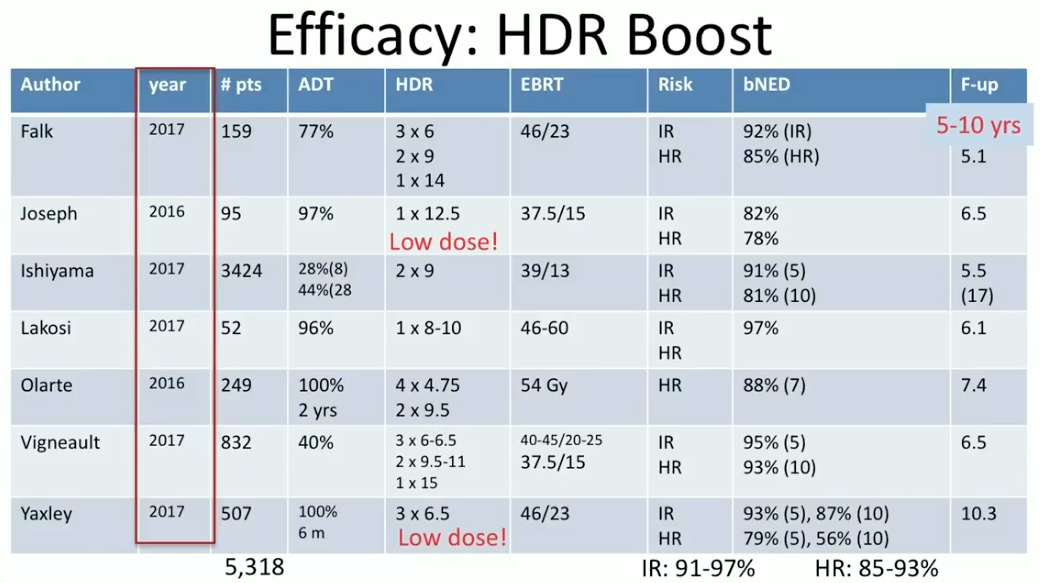
Dr. Crook summarizes efficacy and toxicity results from HDR BT as a boost and as monotherapy from the past 18 months. She reviews seven reports from 2016 and 2017 on the efficacy of HDR BT boost at 5-17 year follow ups. Notably, at the 5-8 year mark, the overall BED rates in intermediate-risk patients were between 91% to 97%, and between 85% to 93% in high-risk patients.
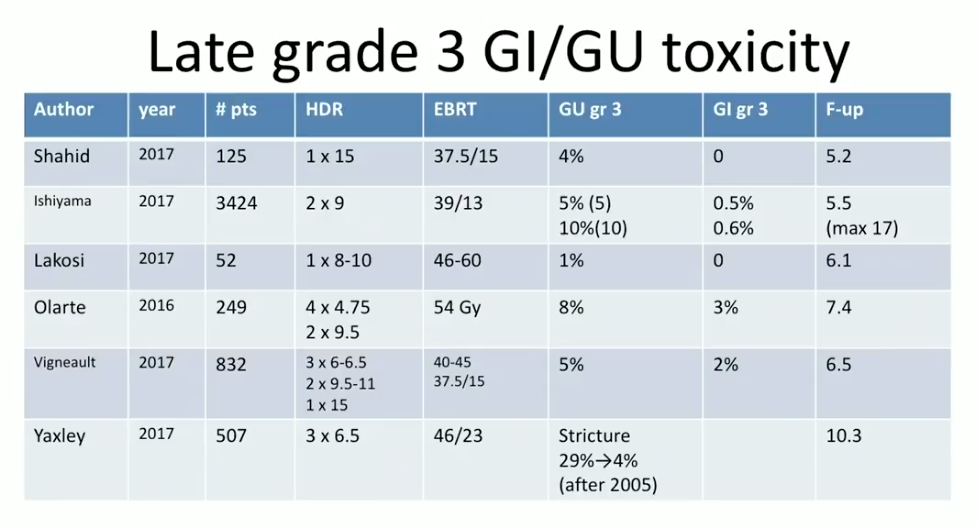
According to toxicity data from these studies, in general, gastrointestinal (GI) grade 3 toxicity rate was between 0% to 3%. The genitourinary (GU) grade 3 toxicity rate was about 5%, however, with occasional higher reports when clinicians treated too much tissue below the prostatic apex.
Subsequently, Dr. Crook reviews HDR BT monotherapy results from 2016 and 2017. This is a much newer method than utilizing HDR as a boost, therefore, these reports are not nearly as mature. Nonetheless, the progression of fractionation data is striking. Also of note, the GI grade 3 toxicity rates from these reports were almost invariably 0%.
Due to this long-term data and the aforementioned technical advantages, Dr. Crook claims that HDR BT is a highly effective treatment with low toxicity.
Optimal Monotherapy Dose
The established dose for HDR BT boost is 15 Gy per one fraction. However, the optimal dose and fractionation for HDR monotherapy is still evolving. This uncertainty may be a result of the fact that the linear-quadratic model for calculating BED is less accurate at faction sizes larger than 10 Gy to 16 Gy. Also, single fraction doses do not have the sensitization advantages that multiple fraction treatment does.
Dr. Crook explains why a single 19 Gy dose may not be optimal, and evaluates the literature supporting either 6 fractions of 7.25 Gy in 2 implants or 2 fractions of 13 Gy to 13.5 Gy as the optimal dose for HDR BT monotherapy. She also discusses an article by Keam et al. that observed patients who received HDR BT with 2 × 10 Gy fractions two weeks apart.
Future Directions
Dr. Crook believes that future research and efforts in HDR BT should focus on incorporating the results of advanced imaging with multiparametric (mp) MRI and mpMRI – transrectal ultrasound (TRUS) fusion into treatment planning and workflow to allow for focal dose escalation to sites of dominant disease.
ABOUT THE AUTHOR
Juanita M. Crook, MD, FRCPC, completed her medical training at the University of Toronto and her Residency in Radiation Oncology at Princess Margaret Hospital. She is currently a Professor of Radiation Oncology at the University of British Columbia in Kelowna. She is a radiation oncologist at the BC Cancer Agency Sindi Ahluwalia Hawkins Centre for the Southern Interior, also in Kelowna, where she has developed image-guided HDR gynecologic brachytherapy, US-planned HDR prostate brachytherapy, and permanent seed brachytherapy for breast cancer.
Previously a Professor of Radiation Oncology at the University of Toronto/University Health Network and Associate Professor at the University of Ottawa, she has a particular interest in intermittent androgen suppression, post-radiation prostate biopsies, and penile brachytherapy. She has written more than 20 book chapters and over 200 journal articles and is a frequent speaker at international meetings where she presents in English, French, and Spanish. She was Scientific Chair of the 2007 American Brachytherapy Society meeting and frequently teaches at the ABS Prostate Brachytherapy School. She is the Former President of the Board of the American Brachytherapy Society and the recipient of the Thom Shanahan Distinguished Brachytherapy Educator Award.