Laurence Klotz, MD, presented “MicroRNA Expression Profiles Identify Clinically Significant Prostate Cancer” during the 29th Annual International Prostate Cancer Update on January 26, 2019 in Beaver Creek, Colorado.
How to cite: Klotz, Laurence “MicroRNA Expression Profiles Identify Clinically Significant Prostate Cancer” January 26, 2019. Accessed Apr 2025. https://grandroundsinurology.com/microrna-expression-profiles-identify-clinically-significant-prostate-cancer/
MicroRNA Expression Profiles Identify Clinically Significant Prostate Cancer – Summary:
Laurence Klotz, MD, discusses a liquid biopsy urine test that uses microRNA expression profiles to identify clinically significant prostate cancer in patients. He emphasizes the ease and efficiency of the technology, in addition to its high degree of sensitivity and specificity.
A New MicroRNA Expression Profile System
There are a plethora of biomarkers currently available for detecting prostate cancer and predicting treatment responses. However, a microRNA expression profile assay from miR Scientific is a platform evaluating intracellular communications rather than evaluating a single biomarker. This platform technology has potential for diagnosis and risk stratification in prostate cancer as well as multiple urologic diseases.
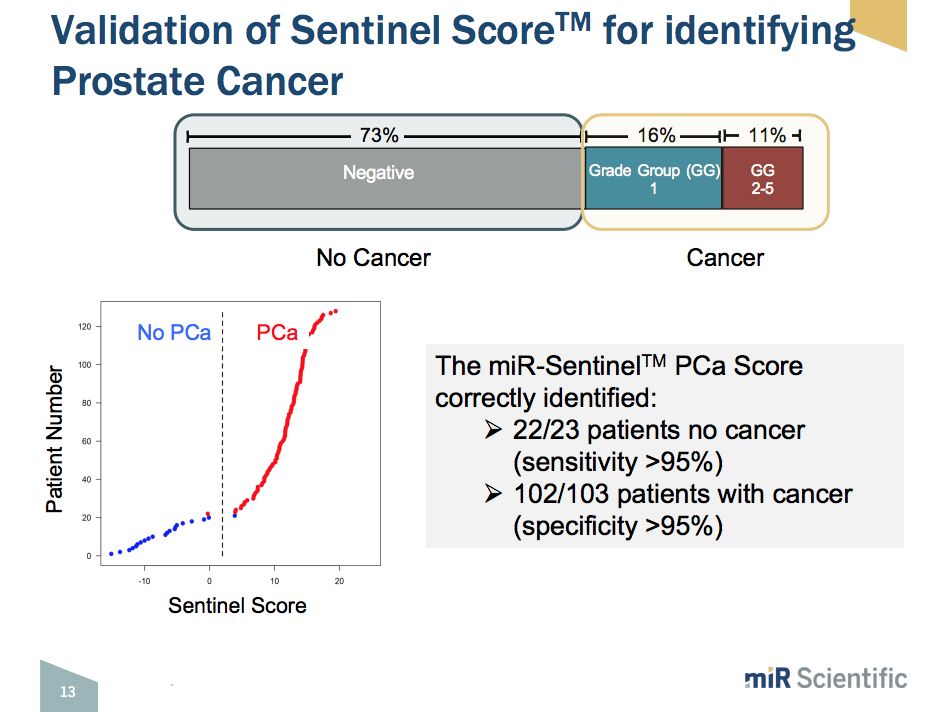
The platform utilizes the thousands of exosomes that cancer cells shed into the urine every day. These exosomes contain contain microRNA, protein, and enzymes. This liquid biopsy urine test uses a panel of around 6,000 microRNAs. There are 50 to 250 microRNAs that predict for the presence of various disease types, stages, and risk categories. In addition to prostate cancer, it can detect information regarding bladder and kidney cancer. Thus, upon the development of a microRNA expression profile, the iterative addition of information from each sncRNA species leads to the calculation of a Sentinel Score™. The Sentinel Score™ measures disease severity and likelihood of having a disease.
This microRNA expression profile assay is unlike other biomarker tests because it does not rely on understanding the function of microRNAs found. Instead, the assay looks for the presence of microRNAs that are the most predictive of cancer.
Initial Data
This microRNA expression profile system is efficient and cost-effective. It has the ability to run around 240 samples per day. Initial trial findings show that the first panel, which simply looks at whether or not patients have prostate cancer, proved to have greater than 95% sensitivity and specificity. The second panel, which looks at clinically significant versus clinically insignificant prostate cancer, proved to have 95% specificity and 93% sensitivity.
MicroRNA expression profiles represent a promising new variation on using biomarkers to identify clinically significant prostate cancer. Also promising is the development of new panels to make the system useful for looking at further cancer sites.
About the International Prostate Cancer Update
The International Prostate Cancer Update (IPCU) is an annual, multi-day CME conference focused on prostate cancer treatment updates. The conference’s faculty consists of international experts, and the event caters to urologists, medical oncologists, radiation oncologists, and other healthcare professionals. Topics encompass prostate cancer management, from diagnosis to treating advanced and metastatic disease. Dr. Klotz presented this lecture during the 29th IPCU in 2019. Please visit this page in order to learn more about future IPCU meetings.
ABOUT THE AUTHOR
Laurence Klotz, MD, FRCSC, is a professor of surgery at the University of Toronto and the Sunnybrook Chair of Prostate Cancer Research. Dr. Klotz was the founding editor-in-chief of both the Canadian Journal of Urology and the Canadian Urology Association Journal (CUAJ), and he is now editor emeritus of the CUAJ. Dr. Klotz obtained his medical degree and completed his residency at the University of Toronto. He was also a uro-oncology fellow at Memorial Sloan Kettering Cancer Center in New York.
Dr. Klotz has 550 peer review publications and eight books. He coined the phrase “active surveillance” and successfully championed this approach for men with favorable-risk prostate cancer against substantial resistance. He was the associate editor of the Journal of Urology, responsible for prostate cancer, for eight years. Dr. Klotz received the Queen’s Jubilee Medal for outstanding public service, the University of Toronto's Lister Prize, the Society of Urologic Oncology’s SUO Medal, the American Urological Association’s Richard Williams Award, the University of Toronto's Lifetime Achievement Award, the Canadian Urological Association Lifetime Achievement Award, and the Harold Warwick Award from the Canadian Cancer Society for “outstanding contributions to cancer control.” In 2015 he was inducted as a Member of the Order of Canada, Canada’s highest civilian award.