Daniel P. Petrylak, MD, presented “Bladder Cancer and Immunotherapy” during the 3rd Annual International Bladder Cancer Update on January 23, 2019 in Beaver Creek, Colorado.
How to cite: Petrylak, Daniel P. “Bladder Cancer and Immunotherapy” January 23, 2019. Accessed Jan 2026. https://grandroundsinurology.com/bladder-cancer-immunotherapy/
Bladder Cancer and Immunotherapy- Summary:
Daniel P. Petrylak, MD, reviews data regarding currently-approved immunotherapy agents for bladder cancer in first- and second-line settings. He advocates for future research regarding immunotherapy-based combinations, as well as investigating immunotherapy in earlier stages of the disease.
Abstract:
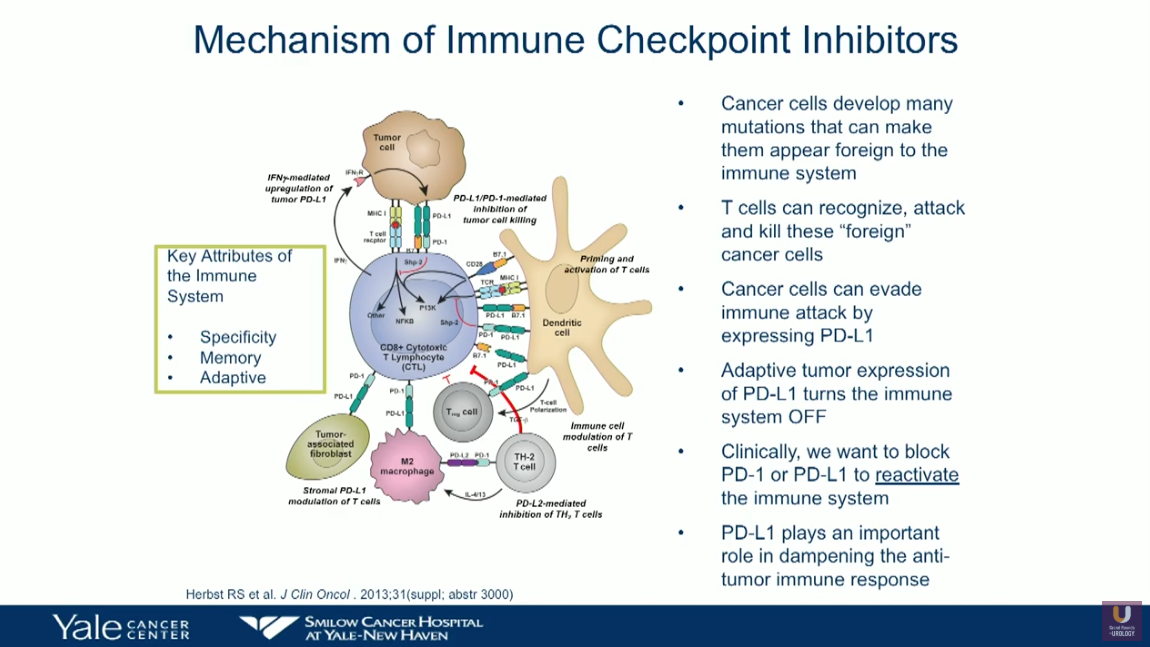
Immunotherapy has emerged as a promising treatment option for patients with bladder cancer. Of the five currently-approved checkpoint inhibitors for urothelial carcinoma (UC), atezolizumab and pembrolizumab have approval in the first-line, cisplatin-ineligible setting, as well as in the platinum-pretreated patients. Nivolumab, durvalumab, and avelumab only have approval for platinum-pretreated patients.
Patient selection for first-line checkpoint inhibition therapy remains a challenge due to inconsistencies in the definition of medically unfit patients. Nonetheless, the treatment goals for these patients has shifted towards palliation of symptoms rather than survival or therapy response. This presentation reviews findings from IMvigor210 (Cohort 1) and KEYNOTE-052 trials as they pertain to checkpoint inhibitors as first-line therapy for cisplatin-ineligible patients.
Results from these trials led to the exploration of potentially moving checkpoint inhibitors to the front-line cisplatin-eligible patients, as well as a restructuring of therapy sequencing for cisplatin-ineligible patients. This presentation analyzes recent data on response rates to checkpoint inhibition in patients with PD-L1 low status, as well as first-line chemotherapy/checkpoint inhibition combination in metastatic UC.
Future research on this topic should focus on the possibility of immunotherapy for non-muscle invasive disease, investigating further therapy combinations, and developing of biomarkers beyond PD-L. Additionally, physicians should maintain a thorough understanding of the markers of resistance and response, as this will help when designing future trials in earlier disease.
About the International Bladder Cancer Update
The International Bladder Cancer Update (IBCU) is an annual one-day CME conference focused on bladder cancer treatment updates. IBCU takes place during its sister conference, the International Prostate Cancer Update (IPCU). The conference’s faculty consists of international experts, and the event caters to urologists, urologic oncologists, and other healthcare professionals. In addition to didactic lectures, IBCU features interactive discussions, a panel roundtable, debates, and case presentations. Dr. Petrylak presented this lecture during the 3rd IBCU in 2019. Please visit this page in order to learn more about future IBCU meetings.
ABOUT THE AUTHOR
Daniel P. Petrylak, MD, is a Professor of Medicine, specializing in Medical Oncology and Urology, and Chief of Genitourinary Oncology at Yale University Cancer Center in New Haven, Connecticut. Dr. Petrylak is an internationally renowned medical oncologist and is considered a pioneer in the research and development of new drugs and treatments to fight cancers of the prostate, bladder, and kidney.