Leonard S. Marks, MD, presented “MRI/US Fusion Biopsy” during the 24th Annual Southwest Prostate Cancer Symposium on April 13, 2019 in Scottsdale, Arizona.
How to cite: Marks, Leonard S. “MRI/US Fusion Biopsy” April 13, 2019. Accessed Jan 2026. https://grandroundsinurology.com/mri-us-fusion-biopsy/
MRI/US Fusion Biopsy – Summary:#
Leonard S. Marks, MD, reviews the utility of MR/US fusion prostate biopsy devices in detecting clinically significant prostate cancer and guiding targeted focal therapy. He also discusses how to develop a MR/US fusion biopsy procedure and integrate its use into a clinical practice.
Abstract:#
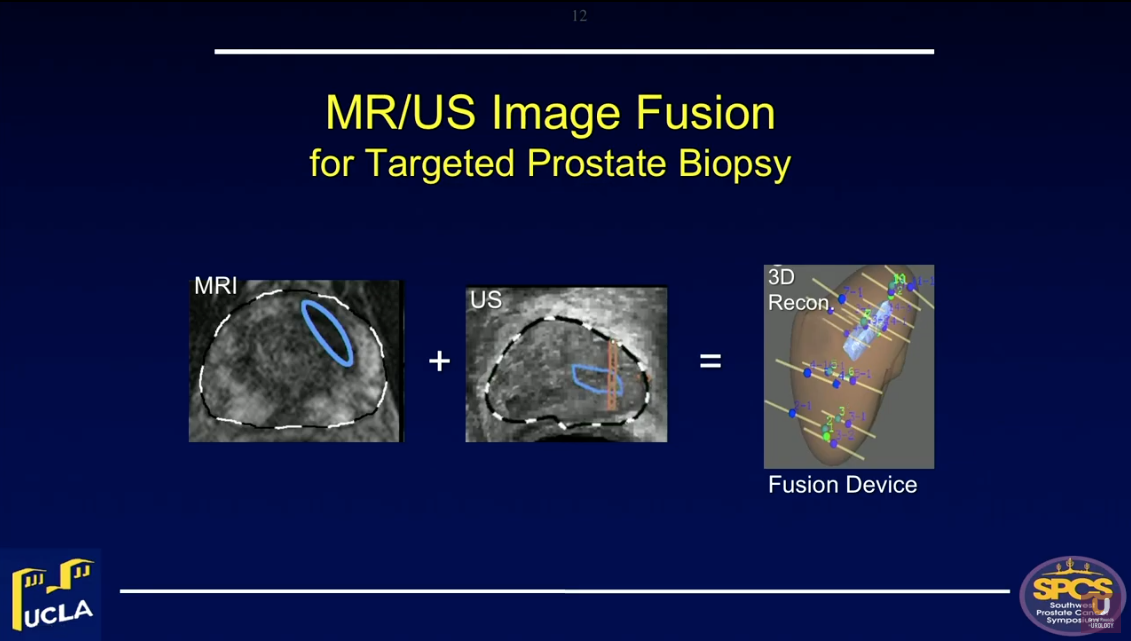
Magnetic resonance/ultrasound (MR/US) fusion prostate biopsy allows for the targeting of suspicious regions, template-mapping for systematic sampling, and tracking of cancer foci over time. All of these functions were not previously possible with US-guided biopsy.
Both the American Urological Association (AUA) and Society of Abdominal Radiology (SAR) have endorsed this procedure for men with prior negative biopsy, but continued cancer suspicion.
Targeted biopsy using MR/US fusion also improves detection of clinically significant prostate cancer (csPCa). Highly suspicious regions of interest on MRI are likely to contain cancer on targeted biopsy. The Prostate Imaging Reporting and Data System (PI-RADS) grading system of MRI is the most important determinant of biopsy outcome. Approximately 25% of PI-RADS Grade 3 lesions, or regions of interest (ROIs), will contain csPCa on biopsy. This detection rate rises to 40% with Grade 4 ROIs and to 80% with Grade 5 ROIs.
Combining targeted and systematic biopsies leads to the maximum likelihood of cancer detection. Physicians should perform both of these when performing MRI/US fusion biopsy for the first time in a man suspected of having prostate cancer. Additionally, the false negative rate of MRI is 15-20% in expert hands. Therefore, physicians should exercise caution when using a negative MRI to obviate prostate biopsy. When a biopsy is indicated clinically, a negative MRI should not preclude biopsy.
Optimally, physicians should perform biopsies using a fusion device capable of tracking biopsy site locations. This function is particularly valuable in men undergoing active surveillance for low-risk lesions. However, when both MRI is negative and PSA density is low (<0.15), the chances of finding csPCa are small (8%).
Fusion devices also provide targeting functions. Biopsy guidance, via MRI localization of cancer within the prostate, provides the foundation for focal therapies of prostate cancer, such as HIFU, cryotherapy, and laser ablation.
About the Southwest Prostate Cancer Symposium#
The Southwest Prostate Cancer Symposium (SPCS) is a multi-day conference that seeks to educate urologists, radiation oncologists, medical oncologists, and other healthcare professionals involved in the treatment of prostate cancer. The topics focus on current technical aspects of diagnosis and treatment of localized and advanced disease, particularly regarding imaging, technology, and training in the related devices. Dr. Marks presented this lecture during the 24th SPCS in 2019. In 2020, the 25th SPCS will also offer training sessions involving imaging, scanning, and prostate cancer treatment related devices on site. Please visit this page in order to register for future SPCS meetings.
ABOUT THE AUTHOR
Leonard S. Marks, MD, is Professor and inaugural holder of the deKernion Chair in Urology at the David Geffen School of Medicine at the University of California, Los Angeles. He graduated from the University of Texas Medical Branch, where he received his MD with AOA honors and his MA in Physiology in 1969. He served an internship and surgical residency at UCLA/Harbor General Hospital. For the following two years, he served on active duty as Lieutenant Commander in the US Public Health Service. He was named a Post-Doctoral Research Scholar at UCLA School of Medicine in 1973-4. He completed his Urology Residency at UCLA and was a Lecturer in Urology there in 1978, following which he entered private practice in Los Angeles. He re-joined the UCLA faculty full-time in 2009.
While in practice, Dr. Marks founded a non-profit research organization, the Urological Sciences Research Foundation, a 501c3 corporation, to further his academic interests. He became an original AFUD scholar in 1992 for work relating serum PSA levels to prostate histology. He received a Prostate Cancer Foundation (PCF) research award in 2000 for cross-cultural studies of prostate cancer. As an early advocate of multimedia in medical education, he served as Website Editor of Urology for The Gold Journal from 1998-2010. For his work in the scientific evaluation of alternative medicines, he was appointed to committees of the American Urological Association, the National Institutes of Health, and the National Academy of Sciences.
Over the past decade, he has been Principle Investigator of three separate R01 Awards from the National Cancer Institute devoted to targeted prostate biopsy and focal therapy of prostate cancer. He has authored more than 150 scientific publications.