How to cite: Andriole, Gerald L.. “Non-Invasive Molecular Imaging – Fluciclovine” October 3, 2019. Accessed Nov 2025. https://grandroundsinurology.com/non-invasive-molecular-imaging-fluciclovine/
Non-Invasive Molecular Imaging – Fluciclovine – Summary:
Gerald L. Andriole, Jr., MD, discusses the unmet need for precise imaging of biochemical recurrent prostate cancer. He reviews data on imaging agents, especially 18F-fluciclovine PET/CT, ¹¹C-choline PET/CT, and 68Ga-PSMA-11, and deliberates on the impact of imaging-guided treatment changes on patient outcomes.
Abstract:
Many prostate cancer patients experience biochemical recurrence (BCR) after treatment. BCR occurs within 10 years in 20-40% of patients who have radical prostatectomies, and in 30-50% of patients who are treated with radiation.
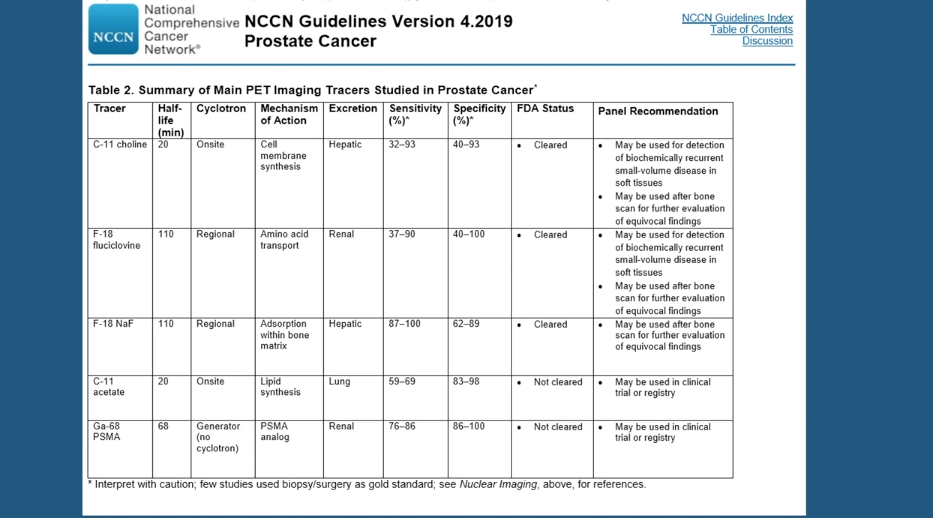
It is often difficult to determine the site of this recurrent disease, and cross-sectional imaging and conventional Tc-99m bone scans are rarely positive in asymptomatic men with PSA < 10 ng/mL. Positron-emission tomography (PET) imaging has shown more promise, though questions remain as to what the most effective PET agent is.
¹¹C-choline PET/CT (choline) and 18F-fluciclovine PET/CT (FACBC) are approved imaging agents for the detection of recurrent disease. Studies show that choline has a pooled detection rate of 62%. However, this agent’s performance varies depending on different factors. Choline has a 36% detection rate for nodal/distant metastases, and only a 25% detection rate for bone metastases. It also has reduced sensitivity and specificity in patients with PSA > 2 ng/mL.
The LOCATE trial found FACBC to have a detection rate of 57%. FACBC has an advantage over choline, however, in its greater sensitivity and superior ability to locate bone metastases. Some researchers have suggested that a third agent, 68Ga-PSMA-11, which is not approved in America, has higher detection rates than FACBC, although some have questioned this study’s validity. Other research indicates that FACBC has an advantage in terms of detecting curable localized disease close to the urinary bladder.
Effective imaging leads to changes in disease management. In the FALCON Trial, ⅔ of patients had a change in management as a result of scanning with FACBC. While It is unclear whether treatment changes affect ultimate outcome, some retrospective studies suggest that metastasis-directed therapy improves survival rates, meaning imaging agents like FACBC may save lives.
About The 4th Global Summit on Precision Diagnosis and Treatment of Prostate Cancer:
The Global Summit on Precision Diagnosis and Treatment of Prostate Cancer is a multi-day, multi-disciplinary forum designated to informing health care stakeholders about topics including in-vitro fluid- and tissue-based molecular diagnostics, novel observation strategies such as active surveillance, and novel therapeutic interventions. Along with this forum’s efforts to form a consensus on the future of prostate diagnostics and precision care, it aims to create an educational and research strategy for its realization. Dr. Andriole presented this lecture during the 4th iteration of this Summit in 2019.
ABOUT THE AUTHOR
Gerald L. Andriole, Jr., MD, is the Chief Medical Officer of Prostatype Genomics AB in St. Louis, Missouri. Dr. Andriole is an internationally recognized key opinion leader in urology with a significant focus on prostate cancer. His research and clinical experience, much of which focuses on genomic testing, allows him to continue to positively impact the quality of care and outcomes for patients diagnosed with prostate cancer.