Michael A. Brooks, MD, presented “Prostate Cancer Genetic Testing: How to Hedge Bets in Selecting Patients for Surveillance, and When to Pull the Trigger on Treatment” during the 24th Annual Innovations in Urologic Practice on September 14, 2019 in Santa Fe, New Mexico.
How to cite: Brooks, Michael A. “Prostate Cancer Genetic Testing: How to Hedge Bets in Selecting Patients for Surveillance, and When to Pull the Trigger on Treatment” September 14, 2019. Accessed Feb 2026. https://grandroundsinurology.com/prostate-cancer-genetic-testing-how-to-hedge-bets-in-selecting-patients-for-surveillance-and-when-to-pull-the-trigger-on-treatment/
Prostate Cancer Genetic Testing: How to Hedge Bets in Selecting Patients for Surveillance, and When to Pull the Trigger on Treatment – Summary:
Michael A. Brooks, MD, reviews the current evidence on the accuracy of multiparametric MRI fusion biopsy in biopsy-naïve patients and patients on active surveillance. He also discusses three commercially-available genomic tests, their validation using biopsy specimens, and performance in active surveillance cohorts.
Abstract:
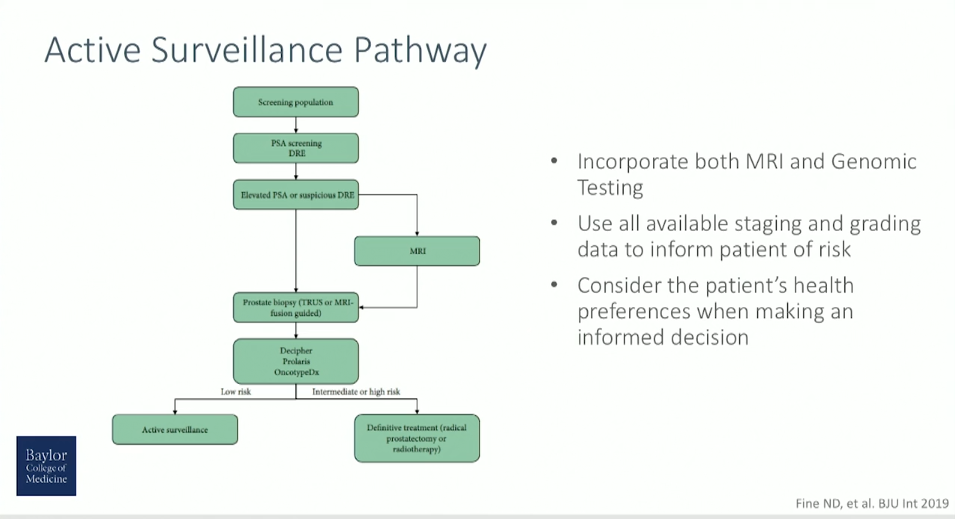
As technology and medicine have evolved, more technologies and methods for accurately risk-stratifying and selecting prostate cancer patients for active surveillance (AS) have become available.
Multiple active surveillance cohorts demonstrate similar long-term outcomes for very-low risk and low-risk prostate cancer. Although definitions of progression vary, treatment-free survival (TFS) remains universally low (15-year TFS 25-55%) with long-term follow-up of all cohorts. Despite high conversion to treatment and strict entry criteria, a small, but appreciable risk of metastatic disease and prostate cancer-specific mortality remains.
Negative oncologic outcomes in patients on AS relate to the under-sampling resulting from traditional prostate biopsy techniques and under-grading resulting from histologic evaluation alone. Using traditional clinical staging and grading, 42-49% of clinically low-risk patients harbor Gleason grade > 6 and 9-16% have non-organ confined disease on pathologic review of prostatectomy specimens.
Multiparametric MRI (mpMRI) fusion biopsy and genomic testing improve long-term risk prediction and patient selection for active surveillance. This presentation reviews the current evidence for mpMRI fusion biopsy in biopsy-naïve patients and patients on AS. Regarding risk stratification, this presentation reviews three commercially-available genomic tests, their validation using biopsy specimens, and performance in active surveillance cohorts.
The presentation concludes with a case presentation demonstrating the clinical utility of both mpMRI fusion biopsy and genomic testing for a patient on active surveillance.
About the 24th Annual Innovations in Urologic Practice
Innovations in Urologic Practice (Innovations) is an annual, multi-day, CME-accredited conference devoted to innovative diagnostic and treatment strategies for and controversies related to some of the most common urologic problems in the current era. The topics covered include oncological management of the bladder, kidney, and prostate. The conference also emphasizes general urology topics in pelvic reconstruction and trauma, men’s health, and infections in the urology patient. Dr. Brooks presented this lecture during the 24th Innovations in 2019. Please visit this page in order to register for future Innovations meetings.
ABOUT THE AUTHOR
Michael A. Brooks, MD, is an Assistant Professor of Urology and Oncology at Baylor College of Medicine in Houston, Texas. Dr. Brooks graduated from Baylor College Of Medicine with his medical degree in 2011. He is also affiliated with the Cleveland Clinic in Cleveland, Ohio. In his practice, he specializes in treating prostate cancer, erectile dysfunction, prostatitis, bladder issues, and other urologic disorders.