Thomas Hope, MD, presented “PSMA-11 PET: Atypical Paths to FDA Approval and Academic NDAs” during the 4th Global Summit on Precision Diagnosis and Treatment of Prostate Cancer on October 3, 2019 in Boston, Massachusetts.
How to cite: Hope, Thomas. “PSMA-11 PET: Atypical Paths to FDA Approval and Academic NDAs” October 3, 2019. Accessed Jun 2025. https://grandroundsinurology.com/current-and-emerging-role-of-radiogenomics/
PSMA-11 PET: Atypical Paths to FDA Approval and Academic NDAs – Summary:
Thomas Hope, MD, Assistant Professor in Residence in Abdominal Imaging and Nuclear Medicine in the Department of Radiology at the University of California, San Francisco, explains how an academic institution successfully submits a new drug application (NDA) to the US Food and Drug Administration (FDA), using the NDA for prostate-specific membrane antigen (PSMA) 11 as an example. He notes the limitations that academia has in this area, and recommends that, going forward, institutions design imaging studies so that they report usable detection rates and have blinded readers.
Abstract:
The process by which an academic institution puts together and submits a successful NDA to the FDA can be mysterious. The NDA submitted by the University of California, Los Angeles (UCLA), and the University of California, San Francisco (UCSF), for Ga-68 PSMA-11 is a good example, and demonstrates how academia could improve its studies to have greater NDA success.
Ga-68 PSMA-11 is a compound that binds the extracellular domain and allows radiologists to see both bone and soft-tissue in one test. It is one of many such compounds, and may not be better or worse than others. However, it has been studied widely by academia because it was not patented. Other compounds are in clinical trials run by pharmaceutical companies. In order to complete an NDA, UCLA and UCSF had to follow the FDA’s 5-module structure, starting with a package insert in module 1 and overview documents in module 2. Modules 3, 4, and 5 focus on chemistry, toxicology, and clinical data reports, respectively. Module 5 was difficult for the researchers from UCLA and UCSF to complete, especially the meta-analysis. Although there are thousands of articles on PSMA, very few report usable detection rates or have blinded readers. Blinded readers are important in imaging studies, because detection rates with one reader tend to creep up, since people are over-incentivized. Academia needs to rethink the design of imaging studies to have more imaging data, although the team from UCSF and UCLA was fortunate enough to have data from fairly reproducible imaging studies, allowing their NDA to be successful.
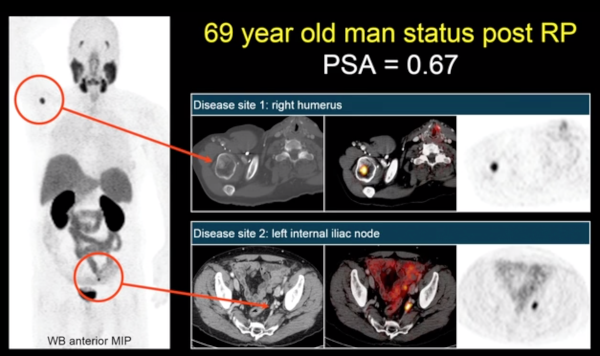
Going forward, UCLA is doing its own major study of PSMA. This is an important step because once these drugs are widely available, a randomized study will be impossible. The success of the NDA is allowing this important research to take place.
About The 4th Global Summit on Precision Diagnosis and Treatment of Prostate Cancer:
The Global Summit on Precision Diagnosis and Treatment of Prostate Cancer is a multi-day, multi-disciplinary forum dedicated to informing healthcare stakeholders about topics including in-vitro fluid- and tissue-based molecular diagnostics, novel observation strategies such as active surveillance, and novel therapeutic interventions. Along with this forum’s efforts to form a consensus on the future of prostate diagnostics and precision care, it aims to create an educational and research strategy for its realization. Dr. Hope presented this lecture during the 4th iteration of this Summit in 2019.
ABOUT THE AUTHOR
Thomas Hope, MD, is an Assistant Professor in Abdominal Imaging and Nuclear Medicine in the Department of Radiology at the University of California, San Francisco. He received his MD from Stanford University and completed an internship at Kaiser Permanente, a residency in Diagnostic Radiology at UCSF, and a Clinical Fellowship in Body MRI and Nuclear Medicine at Stanford. He is the Director for Molecular Therapy. Dr. Hope is the PI on the Ga-68 PSMA-11 IND, has helped lead the development of the clinical PET/MRI program, and is developing the program for neuroendocrine tumors at UCSF. He has published 100+ peer-reviewed articles and is a member of the Radiological Society of North America, the Society of Nuclear Medicine and Molecular Imaging, and the International Society for Magnetic Resonance in Medicine.
