The Role of Molecular Imaging in Managing High Risk and Recurrent Prostate Cancer
How to cite: Reiter, Robert E. “The Role of Molecular Imaging in Managing High Risk and Recurrent Prostate Cancer” January 27, 2018. Accessed [date today]. https://grandroundsinurology.com/The-Role-of-Molecular-Imaging-in-Managing-High-Risk-and-Recurrent-Prostate-Cancer/
Summary:
Robert E. Reiter, MD, summarizes the impact advanced imaging techniques have had on staging and managing prostate cancer in the past few years. He discusses state-of-the-art molecular imaging, particularly focusing on prostate-specific membrane antigen (PSMA) targeted imaging.
Reveal the Answer to Audience Response Question #1
- A. Ga-PSMA PET/CT
- B. Axumin (FACBC) PET/CT
- C. C11-Choline PET/CT
- D. FDG PET/CT
- F. A-D
- G. A-C
Answer Explanation:
FDG PET has very low sensitivity for biochemical recurrence. It might have some utility for advanced disease.
(Twitter Question) Reveal the Answer to Audience Response Question #2
- A. ~75%
- B. ~90%
- C. ~50%
- D. ~10%
Answer Explanation:
Sensitivity for detection of disease (i.e. the number of patients with a positive scan) rises with PSA, but is nearly 50% even for PSA 0.2-0.5.
Reveal the Answer to Audience Response Question #3
- A. PSMA has higher prostate specificity
- B. PSMA only can detect both soft tissue and bone lesions
- C. PSMA probes have a shorter half-life
- D. PSMA probes are less sensitive than choline or axumin
Answer Explanation:
Only PSMA is prostate specific. The others are increased in cancer, but are metabolic markers and not exclusively increased in cancer.
Reveal the Answer to Audience Response Question #3
- A) Immediate hormones and radiation
- B) Immediate radical prostatectomy
- C) Additional molecular imaging with axumin, PSMA or choline (depending on which is available)
Answer Explanation:
Although the impact of improved staging on outcome is not known, this patient has a high likelihood of occult metastatic disease. A positive scan in bone or retroperitoneal lymph nodes, in particular, would likely alter management.
The Role of Molecular Imaging in Managing High Risk and Recurrent Prostate Cancer – Transcript
Click on slide to expand
Molecular Imaging in High Risk and Recurrent Prostate Cancer
All right. I’m going to try and hopefully get us back more on time after that last presentation, but it was actually a great presentation. My task is to talk about the state-of-the-art in molecular imaging. It’s really a huge topic. We actually had a whole session on it at the SUO meeting in Washington. So I’m really going to just give it a kind of cover the surface, really focusing on PSMA imaging in particular.
Imaging in Prostate Cancer by Clinical Stage
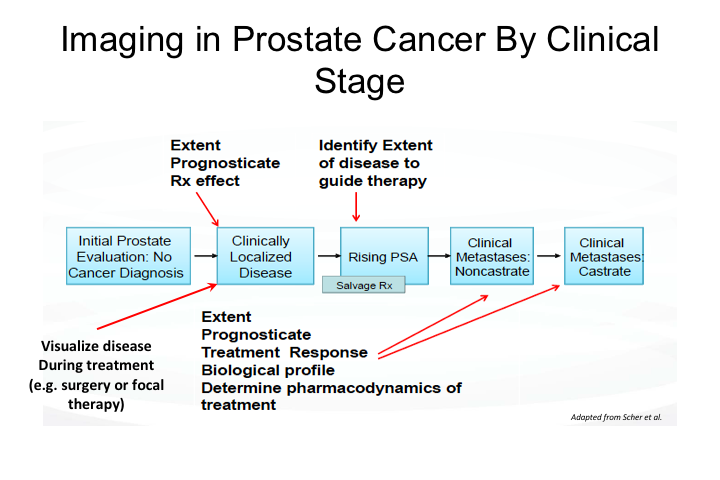
So imaging in prostate cancer. I think probably one of the maybe revolution, but certainly a great evolution, in our field over the last few years has been that we have gone from being a disease, prostate cancer has gone from being a disease that we managed to a certain extent blindly with very poor imaging, difficulty staging, and that ranges from both biopsy, all the way to very advanced disease, to really a disease that we are increasingly able to visualize, and then manage really like most other malignancies are managed in medical and surgical oncology, that is by integrating the location and extent of disease, being able to monitor response to treatment, etc. etc. And so we’re getting there.
And as you can imagine, the entire range of prostate cancer, the entire landscape of prostate cancer management, there are clear needs for imaging. We talked yesterday about MRI for initial prostate evaluation and biopsy and surgical and active surveillance management decisions. But of course we very commonly face particularly as surgeons, the issue of rising PSA and whether to offer salvage radiation or systemic therapy or a combination. In the setting of metastatic disease, we have this whole state of disease called M0, which implies lack of metastatic disease, but is that truly M0 or is it simply our inability to detect the extent and location of disease? And we really have had to a certain extent very poor ways of monitoring disease with therapy. Bone scan, we all know the limitations of any type of bone scan for monitoring disease response because of what a bone scan typically measures, which is bone metabolism rather than actually disease presence or absence, and so these were all areas for which imaging is required to improve our management of the disease.
Molecular Imaging Tracers 2018
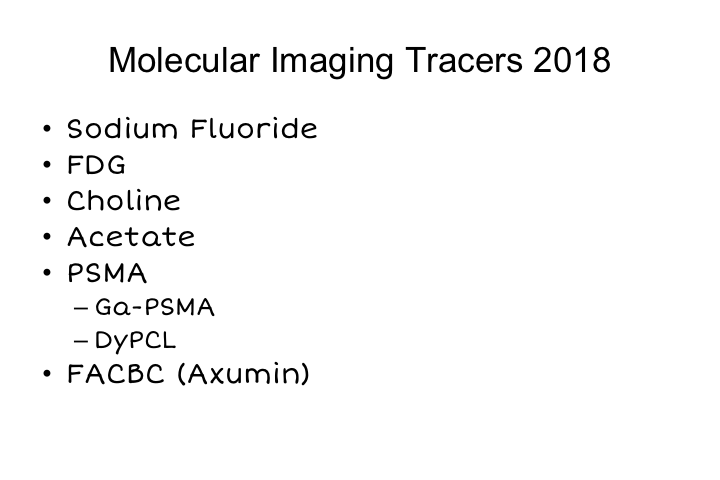
As you can see, there has been really a dramatic expansion in the number of molecular imaging tracers that are available either in the U.S., Europe, or around the world. Not every agent is available everywhere, but I think that is really going to change pretty rapidly over the next few years.
Sodium fluoride PET is fairly widely available. And covered by some insurance companies. FDG PET has been around forever. Choline PET has been around in a number of centers. Was approved for Medicare payment at the Mayo Clinic. Acetate, we were using that at UCLA for many years. Really, most of the work in the field really now is focused on PSMA, and there are a number of different tracers that can be used to essentially monitor PSMA expression in a patient, and then recently approved, and therefore paid, for particularly by Medicare is axumin, which is FACBC which is an amino acid analog, which is sensitive for the detection of prostate cancer as well.
PSMA as a Theranostic Target
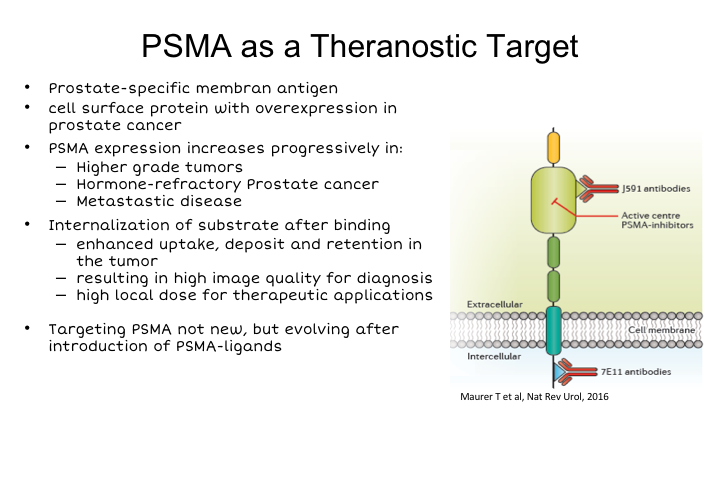
So I’m going to talk mostly about PSMA today, because I think it’s really gained the widest distribution around the world. I think this is where the field is going even if it is not quite there yet in the U.S. and I think that you know this is what is critical to understand. So PSMA is certainly not new and the concept of imaging PSMA is definitely not new, it’s been around for more than 20 years. PSMA is prostate specific membrane antigen. It’s a cell surface protein. We all remember prostasin, the antibody that was developed as an imaging agent and approved probably about 20 years ago, I think when I entered the field.
So the concept is not new, but the agent and the imaging technology 20 years ago certainly was not what it is today. Importantly PSMA is not only fairly prostate specific, but its expression increases in higher-grade tumors. It’s inversely expressed in relationship to androgen. That is it is suppressed normally by androgen, so androgen suppression leads to an increase in PSMA, which is a unique characteristic of it. It is present in virtually all disease, including metastatic disease. It is probably expressed in about 90% to 95% of all prostate cancer. Only very advanced neuroendocrine, or small cell carcinomas, generally don’t express it. It’s internalized after binding. It’s an enzyme, so it has been uniquely suitable either for producing antibodies that could be internalized, or small molecules that could bind to the enzymatic pocket of the enzyme, of PSMA and be used for imaging purposes. Both of those strategies have been pursued with success over the last few years.
Rationale for PSMA Targeted Imaging
So the rationale, obviously, is that our current cross-sectional imaging with CT, MRI, etcetera, etcetera, is either not sensitive or it’s not specific for prostate cancer. PSMA PET provides a prostate, prostate cancer specific target that therefore might provide better sensitivity and should provide very high specificity importantly, which is as a clinician probably more important almost than sensitivity because what we really want to know is if we see something is it real? Can we actually bank on it? Can we make management decisions based on what we see?
PSMA Targeted Imaging Modalities
So as I mentioned, there are a number of different ways to target PSMA. I’m going to really focus on Ga-PSMA down below. Prostasin was developed many years ago. The problem was it recognized an intracellular epitope of the protein, so it could only recognize cells that were necrotic or dead. J591 is an antibody developed Neal Bander‘s group many years ago at Cornell. It recognizes the extracellular domain, and then that antibody has actually been re-engineered by a company that I’m involved with, but I’m not going to speak about today to produce what is called IAB2M, which is basically a minibody or engineered antibody fragment that has the same binding characteristics of J591 and functions quite well, and then small molecules such as Ga-PSMA and DCFPYL, which is being used in a number of clinical trials and is being used actively at Johns Hopkins where it was discovered.
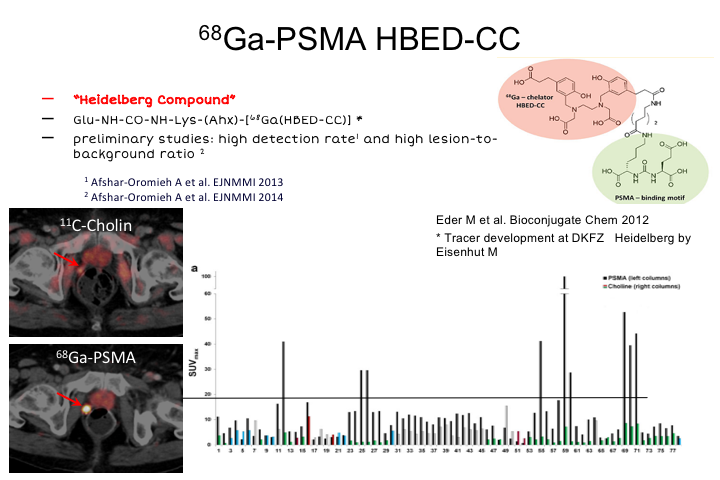
68Ga-PSMA HBED-CC
So Ga-PSMA HBED-CC, it’s also called the Heidelberg compound. Like many of these compounds, it has a urea substrate which is active and binding to PSMA. This was developed in Heidelberg by a group of nuclear medicine and chemistry doctors there and professors. Preliminary studies showed a very high detection rate and high lesion to background ratio. Ga is suitable for PET, because it emits positrons, and is one of a number of different radioisotopes that can be used. It’s fairly easy to work with, but you do have to have a generator on site to produce this. They cost about $200,000. Pretty widespread around Australia and Europe right now, but that has certainly been at least one impediment to taking this up, and some of the new tracers use things like F18, which have much better potential for distribution and production at a central facility, and then ship in to individual sites. So I think the future probably will not be Ga, although the data here will show kind of where we’re going, but probably something like F18, one of the newer molecules that is coming around, and the group in Heidelberg working, which collaborates extensively with our group at UCLA has actually developed an F18 compound very similar to this that looks quite exciting and has some better characteristics, compared to Ga-PSMA.
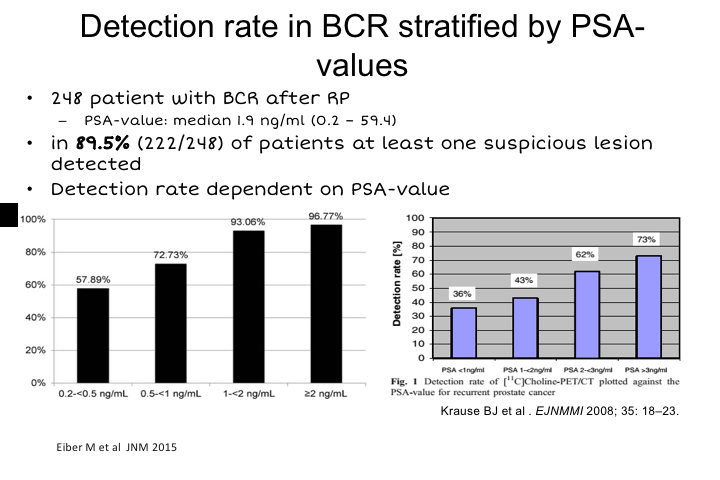
Detection rate in BCR stratified by PSA-values
So the first use that this was developed for and axumin is also approved for this use is for biochemical recurrence, basically to detect the location of disease after failure either from radical prostatectomy, radiation, cryotherapy or whatever, really to localize whether disease is in the prostatic bed, whether it’s regional, whether it’s metastatic to bone, or soft tissues above the pelvic brim.
And overall, in the first study this is a study from Matt Eiber in Germany, 248 patients after biochemical recurrence. Median PSA value in the study was 1.9, and they found at least one suspicious lesion in 90% of patients and then you could stratify the sensitivity for detection, not necessarily for the total number of lesions in a patient, but just on a per-patient basis. You could stratify based on PSA, and as you can imagine, the higher the PSA, the more tumor volume, the more sensitive any radiotracer is going to be. But really pretty impressively, 60% of patients even with PSAs, as low as 0.2 to 0.5, had detectable lesions. There was a significant drop off less than 0.2, probably 10 to 20% but it has really not been studied as well and most protocols require that the PSA be greater than 0.2 in order to have one of these imaging studies done.
And if you compare this to previously published data on choline, you can see that PSA per PSA, PSMA Ga PSMA is more sensitive for the detection of any recurrence compared to choline, and there haven’t been any direct comparisons to axumin yet. We have a lot of experience at UCLA using both, and overall the sensitivity to PSMA appears to be significantly higher than with axumin, but that needs to be proven prospectively, and we’re doing a study right now.
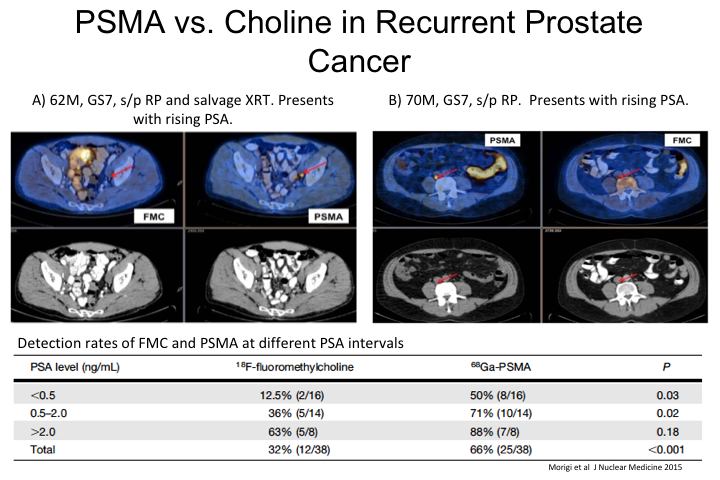
PSMA vs. Choline in Recurrent Prostate Cancer
So, this was actually a direct head-to-head comparison of PSMA versus choline, which before PSMA was really the standard of care throughout Europe and in many centers in the United States. So, these were individual patients imaged with both tracers and so there was a direct comparison as you can see here PSMA PET had a significantly higher sensitivity for the detection of a positive lesion or positive site compared to choline at almost all PSA levels, but particularly at the low PSA levels, 0.5 to, I would say 2. Once you get to very high PSAs, they are both quite sensitive for at least the detection of some disease, although even then, PSMA appears to have higher sensitivity for the number of lesions compared to choline. Similar results have been published with acetate and some of the other tracers.
Performance of Ga-PSMA PET for BCR
This is a meta analysis that was published out of Australia, where they have been crazy about this for the last number of years, and this looks at multiple studies that have been published, and overall what I’ve shown holds true in a meta analysis, which is that the ability to detect biochemical—the site of biochemical recurrence correlates with the level of PSA, and that overall about 50% of men who have PSAs 0.2 to 0.5 have some detectable disease, and as PSA gets higher obviously that number is much higher, and so this was I think a landmark publication in this field.
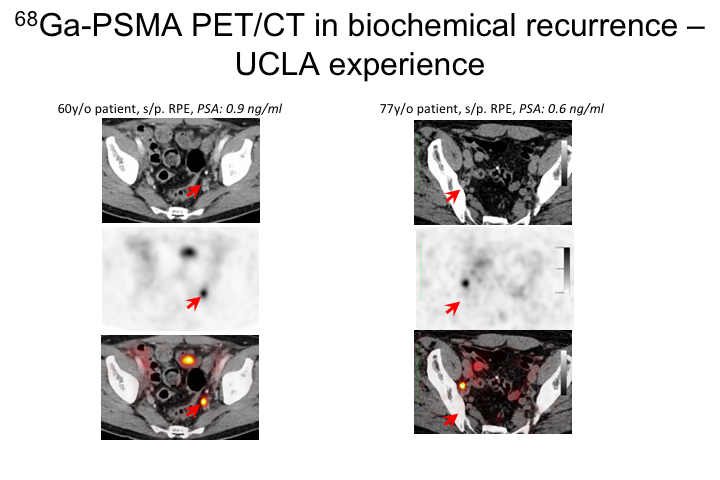
Ga-PSMA PET/CT in biochemical recurrence-UCLA
Here are a couple of cases of my own from UCLA where we have been doing this for about a year and a half, and we do this under an IND so it’s important to know that at least in the U.S. this is not an FDA approved agent. It’s being done in a number of centers. It’s being done under an IND, that allows us to image over 2,000 men, and so it is part of a registry and the hope is that with that information, this will be able to be taken to the FDA for approval and then hopefully payment by Medicare and other insurance companies.
So this is a 60-year-old patient, PSA 0.9 after radical prostatectomy. Had a PSMA scan. You can see here he has this significant node right here, and then this is another guy, 77 years old with a PSA of 0.6, and he also has what I think was an obturator node that was positive. I’ll show a couple of cases during the remainder of the talk to really walk through some of the management options.
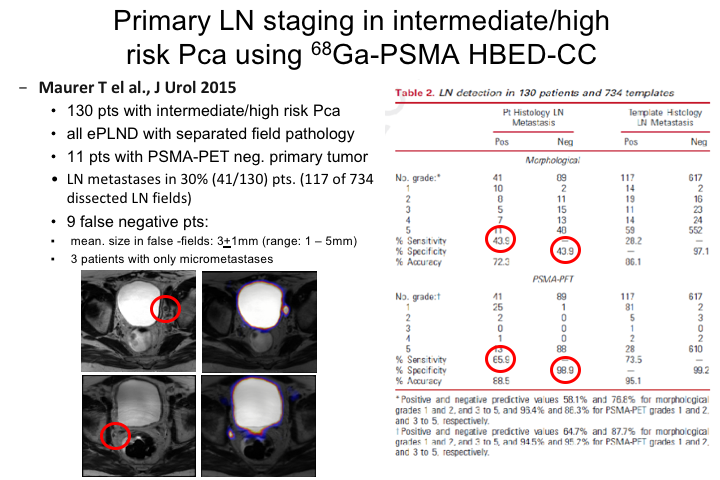
Primary LN staging in intermediate/high risk Prostate cancer using Ga-PSMA HBED-CC
This is actually the first talk. I gave you a new talk yesterday, so we’ll get to the cases at the end. I rearranged things a little bit. So I’ll show you another biochemical recurrence case a little bit later, and I’ll show you some data about how PMSA imaging impacts management because it is great that we can image and see stuff, but at the end of the day how do we use this information, how do we incorporate it into what we currently do, and can we change what we do based on this imaging just yet or do we?
So the other obvious place to use a better imaging agent is for staging. As you know, we’re all seeing much more high-risk disease at this day and age. Extended pelvic lymph node dissections are the rage and probably I think required. And the reason that we, but we really have lacked the ability to detect metastatic, particularly regional disease at the time of diagnosis, so an obvious use is for primary lymph node staging or metastatic or for the detection of metastatic disease in intermediate to high-risk disease. The data here has not been as robust, nor is it as pervasive as it has been for biochemical recurrence, but it certainly has some utility and I think we’ll learn more about this over the coming years.
This was an initial study by a German group, 130 patients with intermediate to high-risk cancer. All patients had an extended pelvic lymph node dissection so that there was—they could estimate the true specificity where that is the false positive or false negative rate of PSMA PET. They were able to detect lymph node metastases in 30% of patients and in 117 of 734 dissected lymph nodes. There were nine false negative patients and importantly the mean size of the tumors are the tumor deposits in the ones who had false negatives were very small 3 plus or minus 1mm. So again getting to the point that PSMA, any PET scan just based on the limitations of PET technology will not detect very much under 5mm in size, so what you are really detecting are things greater than 5mm, so you have to recognize that even with this improvement, we may not be seeing everything. We are seeing hopefully more than the tip of the iceberg, but we are still seeing only a part of what is really going on totally. And so the estimated sensitivity for this in this first study was about 40 or 50%. And that’s been replicated in a number of other sites including our own.
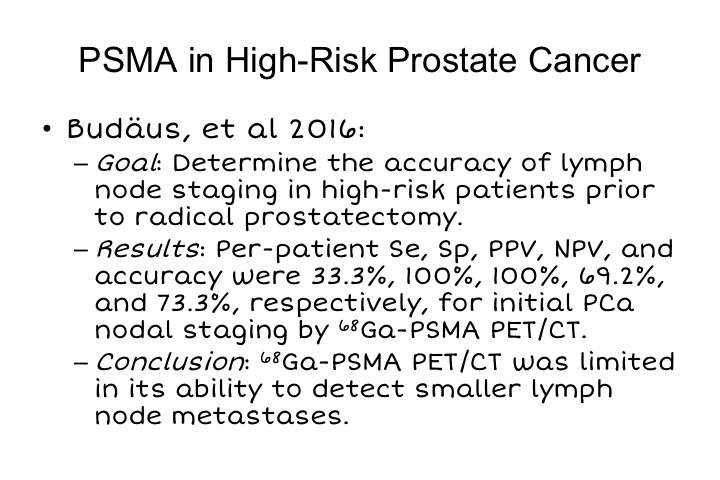
PSMA in High-Risk Prostate Cancer
This was a study that was not as positive. This was done in the Hamburg group at the Martini Clinic, and here they did a study in patients with high-risk disease prior to radical prostatectomy and found that really there was only sensitivity for detection of the presence of disease on a per-patient basis in a third of patients, so it is certainly better than what we have been able to do heretofore, but it suggests that there are still some ways to go in terms of using this for staging, and we are exploring this actively in a prospective study at UCLA and in many other sites around the world. The major limitation, again, is that many of these early lymph node metastases for high-risk disease are simply very small and are below the level of detection. And so that is that study.
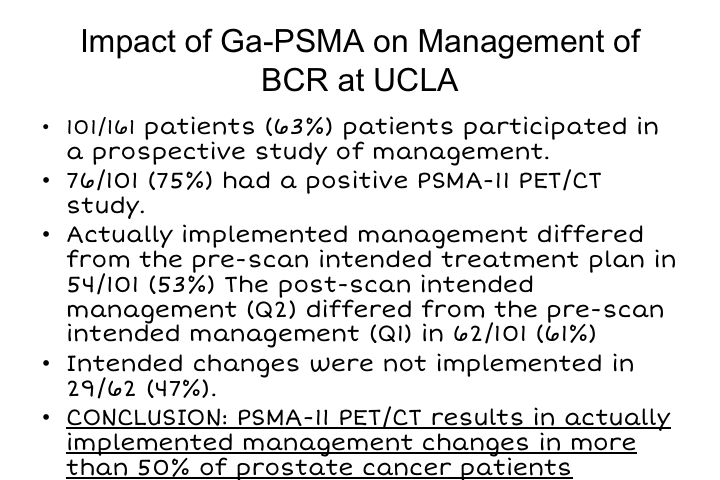
Impact of Ga-PSMA on Management of BCR at UCLA
We just published a study or it was just accepted, and should be published soon, where we really looked at what is the impact of all of this on how we manage biochemical recurrence. So this is a study of the first 161 patients at UCLA that were studied with biochemical recurrence. 101 patients participated in the study. And that involved the physicians filling out questionnaires, both before and after imaging in terms of what their intended therapy was going to be versus what they actually did after they had the information from PSMA. 76 of the 101 patients who participated had a positive PSMA PET scan, and the actually implemented management differed from the pre-scan intended treatment in 54 patients, 53% of patients. So that is pretty significant that in 53% of these patients what was found on PSMA imaging changed and was implemented by those physician in about 50% of patients, and so the conclusion from this study, which should be published shortly was that this resulted in implementing management changes in more than 50%. Now, whether those changes were appropriate is something that really needs to be studied, and I think really where this field needs to go is not just focusing on sensitivity and specificity but really focused on the questions of how does this impact management and are those changes clinically relevant and clinically effective. If we are going to start abandoning doing salvage local radiotherapy because we only see a lymph node, the question is is that appropriate or not, and by the same token should we abandon doing surgery and extended pelvic lymph node dissections if we find regional metastases at the time of diagnosis, and I think those are all unanswered questions, but we should be able to address them prospectively.
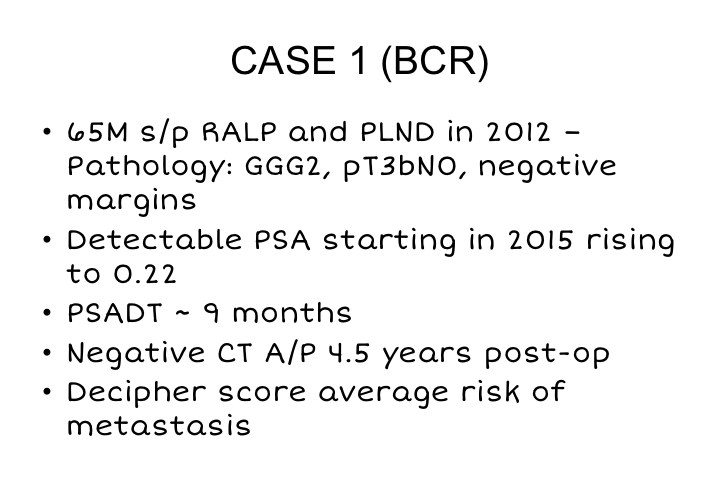
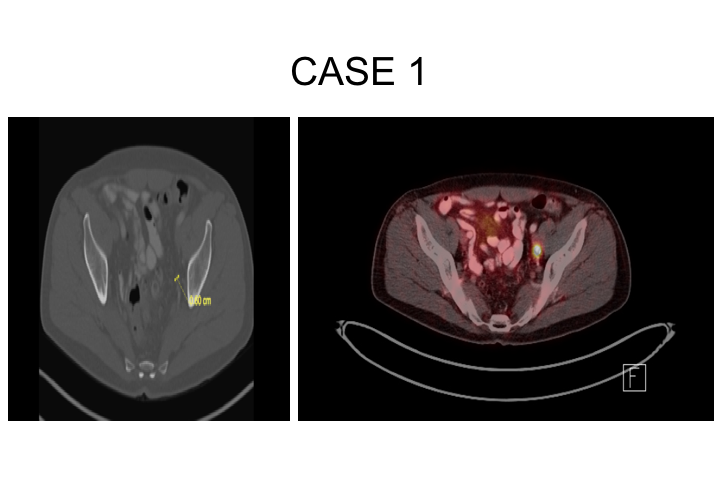
CASE 1 (BCR)
So a couple of cases before I finish. A 65-year-old man who had a robotic prostatectomy in 2012. His pathology showed Gleason grade group 2, seminal vesicle invasion T3B, he had a detectable PSA starting in 2015 so three years post-op, which rose to 0.22, the PSA doubling time was 9 months. He really did not want early salvage radiation, still sexually active and just didn’t want anymore therapy. So because his PSA doubling time was fairly slow, we elected to monitor him, and as soon as his PSA got to be greater than the threshold at which we can actually order a scan, we decided to go ahead and order a PSMA scan. His Decipher score didn’t really help us much. It showed an average risk of metastasis, and all of his standard imaging including acetate scans were negative.
Case 1
So this is the patient here, and you can see he has got this single lymph node. This is actually kind of a residual obturator lymph node that was found, and he elected to undergo surgery to remove this together with all remaining nodes in the pelvis on both sides of his body.
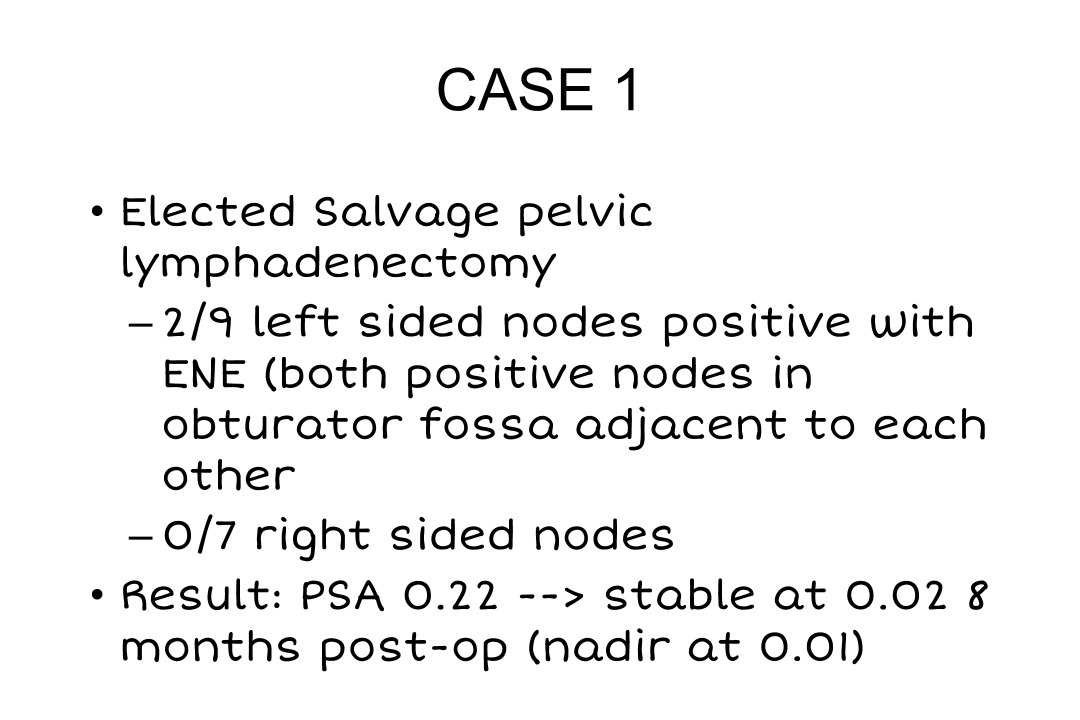
Case 1
What this showed is that—he had had a lymph node dissection before so obviously he didn’t have an extended pelvic lymph node dissection because he was thought to have a relatively low-risk disease, Gleason grade 3+4, and seminal vesicle invasion at that time was not anticipated. So he had two, not one, two lymph nodes that were positive with extranodal extension in the obturator fossa, really basically stuck right to the obturator nerve, it took some doing just to get them released, and no nodes positive on the other side. His PSA has declined to 0.01, 0.02, where it remains at 8 months. Now, time will tell. I’ve spoken to him about doing some additional radiation because I am concerned that he could still have some residual disease in the site of extranodal extent right along the obturator nerve, and we will probably do that if there is any evidence that this is rising.
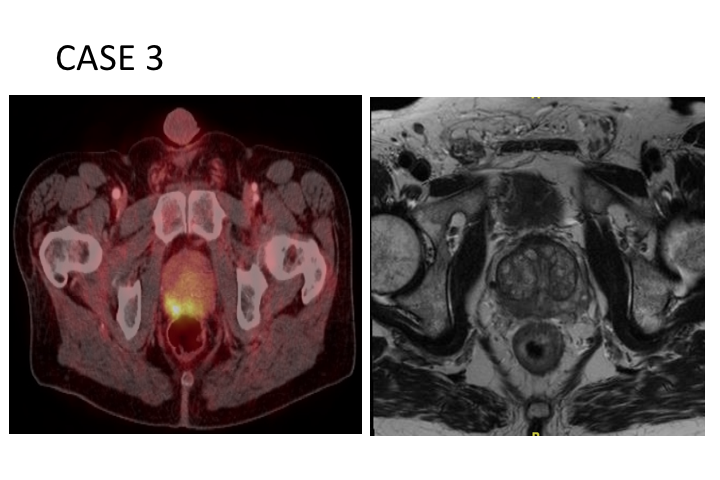
Case 3 (High Risk)
Then here is a high-risk case, a 63-year-old man with Gleason grade group 5, PSA of 8.3, prostate MRI showed Prostate only disease, but on the CT scan there were two suspicious borderline pelvic lymph nodes. So the question is are those real or not. They were not thought to be very amenable to biopsy, so we did order a PSMA as part of our high-risk protocol, and that showed only prostate, only imaging in his prostate, and those two suspicious lymph nodes were actually PSMA negative.
Case 3
So here is his disease in the prostate on the right side. PSMA is actually quite sensitive for detection of primary disease, particularly significant Gleason grade 7 or higher disease. This also is being studied prospectively and has been used as an adjunct in a couple of really extreme cases where an MRI showed something or showed nothing, PSMA PET was positive and we’ve done targeted biopsies using the PET image fused together with the MRI, of course there are MRI PET machines coming onto the market. I can’t imagine how expensive those would be to do, so I am not recommending it routinely. So that is his prostate.
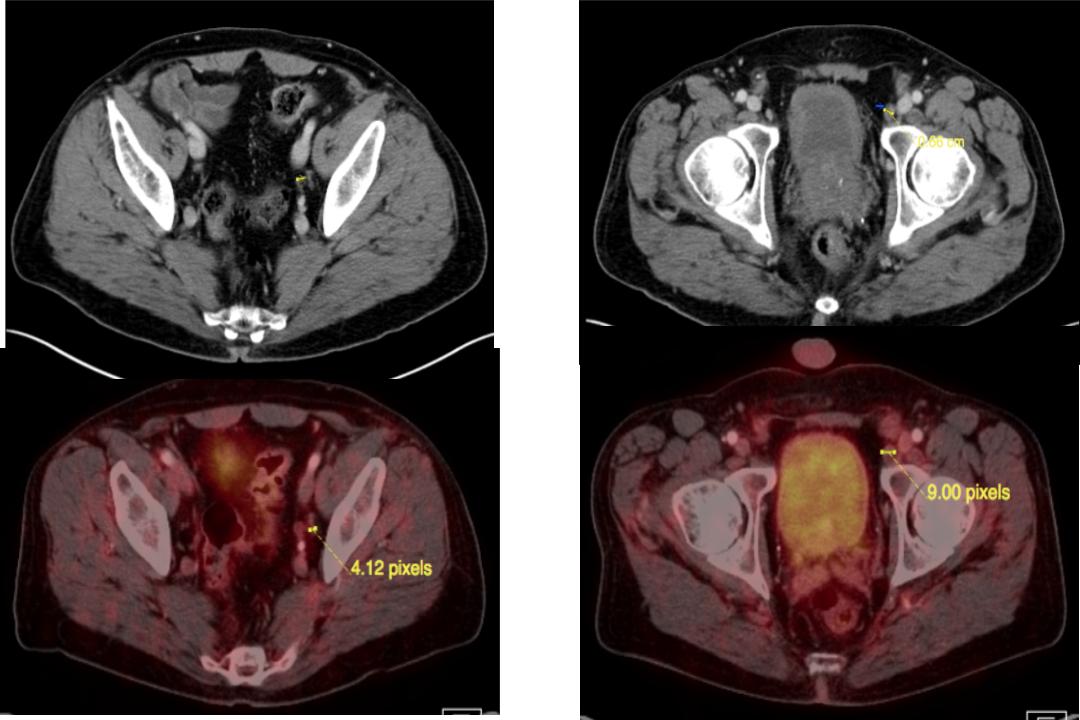
Two Negative PSMA Nodes
Then the site of these two nodes that was found on CT both were actually negative on PSMA. So he underwent surgery because of that.
Case 3
Because of that he had about 30 lymph nodes removed, all of which were negative, and his PSA has been zero at nine months, suggesting that those two nodes that are negative are true false negatives, so I think this is actually the absence of findings that has actually helped his management.
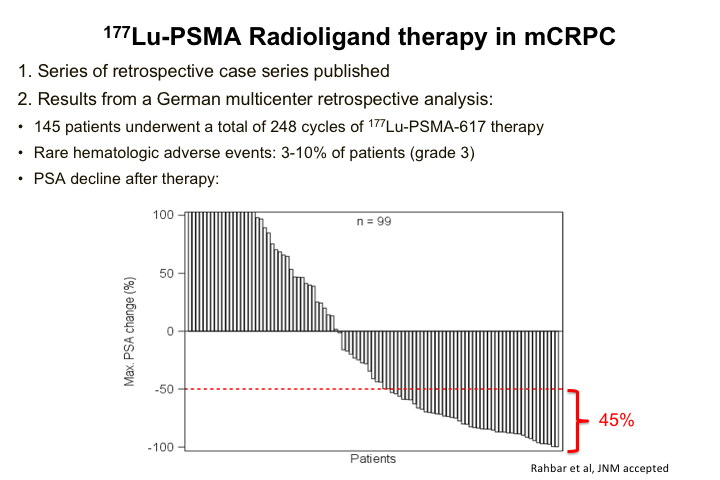
177Lu-PSMA Radioligand Therapy in mCRPC
Then the final slide is really just kind of where things are going. The real excitement in the field is can we actually do a targeted instead of Radium-223, which is targeted to bone in general, can we actually do targeted radiotherapy with so-called radioligand therapy. There have been a number of studies in Germany, generally Phase 1, early Phase 2, that have shown pretty promising results. These are heavily pre-treated patients in general although it’s kind of all comers, kind of a mishmash, so that is why others are now doing more regimented, traditional phase 2 types of prospective studies. But nevertheless about 50% of patients treated with this have had PSA responses, and about half of those have had pretty significant drops in PSA, and so people are quite excited about this. We just opened a Phase 2 prospective trial of this therapy at UCLA. It’s also opened at a center in Houston, and so there is a lot of excitement about this as well. I won’t go through these, it’s just a couple of patients that we treated. Those are open INDs.
ABOUT THE AUTHOR
Robert E. Reiter, MD, MBA, is the Bing Professor of Urology and Molecular Biology and Director of the Prostate Cancer Treatment and Research Program at the David Geffen School of Medicine at the University of California, Los Angeles. He is currently the Principal Investigator of UCLA’s SPORE (Specialized Program in Research Excellence) program, a $12 million research grant from the National Cancer Institute to develop new diagnostic and treatment options for men with prostate cancer. Dr. Reiter’s clinical interests include robotic surgical management of prostate cancer and the use of both MRI and molecular imaging tools to manage this disease. His research is focused on the development of novel antibodies for both treatment and imaging of prostate cancer, as well as on the role of epithelial to mesenchymal transition in castration and treatment resistance. Dr. Reiter completed his undergraduate studies at Yale University and earned his medical degree at Stanford University Medical School.