Dr. Gerald L. Andriole, MD, presented “Updates on the ERSPC and the PLCO Trials” at the 2nd Annual International Prostate Cancer Update on January 27, 2018 in Beaver Creek, Colorado
How to cite: Andriole, Gerald L. “Updates on the ERSPC and the PLCO Trials” January 27, 2018. Accessed. https://grandroundsinurology.com/Updates-on-the-ERSPC-and-the-PLCO-Trials/
Summary:
Dr. Gerald L. Andriole, MD, reviews the designs, materials, and outcomes of the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial (PLCO) and The European Randomized Study of Screening for Prostate Cancer (ERSPC). He distinguishes between legitimate concerns and inappropriate criticisms of the trials. Also, he explains what this means for the practice of PSA testing.
Updates on the ERSPC and the PLCO Trials – Transcript
Click on slide to expand
Reveal Answer to Audience Response Question #1
Does age-based PSA screening reduce overall mortality?
- Yes
- No
Answer Explanation:
While most of the people in the audience answered, “Yes,” I think most people would say there’s no way we’re going to improve overall mortality, because the fraction of the population that dies of prostate cancer is so small.
(Twitter Question) Reveal Answer to Audience Response Question #2
Does age-based PSA screening reduce prostate cancer-specific mortality?
- Yes
- No
- Maybe
Answer Explanation:
The answer is either yes or maybe. It really depends on how you look at the data.
Human Nature
I saw this on Twitter, and this was from Bertrand Russell about 100 years ago, and I think it applies exactly to prostate cancer screening. “If a man is offered a fact which goes against his instincts, he will scrutinize it closely, and unless the evidence is overwhelming, he will refuse to believe it. If, on the other hand, he is offered something which affords a reason for acting in accordance with his instincts, he will accept it even on the slenderest evidence. The origin of myths is explained in this way.” And I think this is exactly—you could look at the PLCO and the ERSPC data and find exactly what you want.
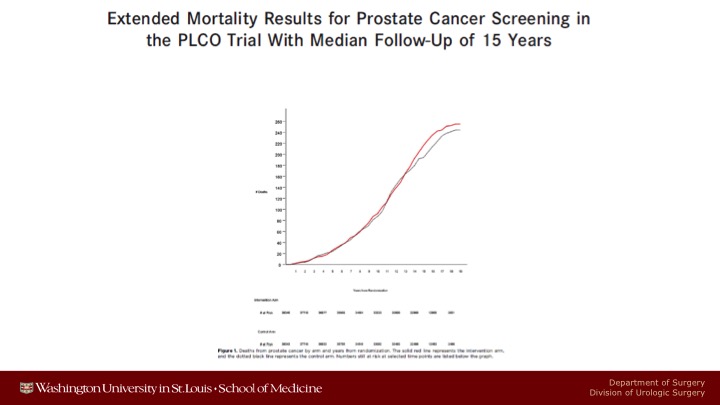
Extended Mortality Results for Prostate Cancer Screening in the PLCO Trial With Median Follow-Up of 15 Years
We’ve published PLCO data three times, most recently just last year.
Mortality Results from a Randomized Prostate-Cancer Screening Trial
And we continue to show absolutely no prostate cancer specific mortality benefit with 15 years of follow up.
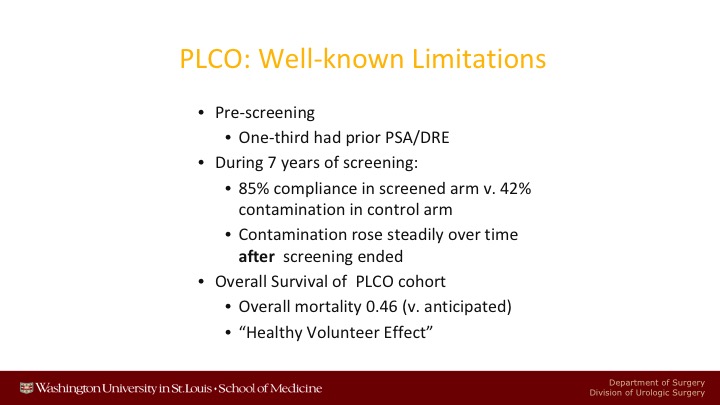
PLCO: Well-known Limitations
There are many well-known limitations of the PLCO trial. There was significant pre-screening. During the seven years of screening, there was 85% compliance in the screened arm versus 42% contamination in the control arm. So it was a comparison of pretty aggressive screening versus partial screening. After the 7 years of screening of PLCO was over, contamination rose steadily, and up to 90% of the patients ended up getting screened in the control arm.
The main other point about PLCO is that the overall survival of these patients was much, much better than was anticipated when the study was designed. In fact, only half as many patients died after 15 years as was anticipated would have died, or otherwise end pointed so that you could have enough endpoints.
Reevaluating PSA Testing Rates in the PLCO Trial
These are just some of the well-known data showing the escalating incidence of contamination in the control arm.
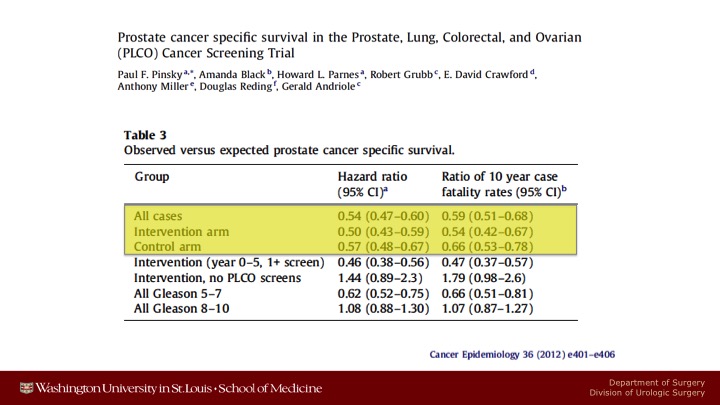
Prostate cancer specific survival in the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial
These are data showing that there were only half as many prostate cancer deaths in either arm as was anticipated at the time.
Inappropriate Criticisms of PLCO
Now, having said that those are legitimate criticisms of PLCO, there are some criticisms of PLCO that have been out there and that are improper. And one of them is that there was a low biopsy rate and that because of this low biopsy rate, we missed a chance for cure.
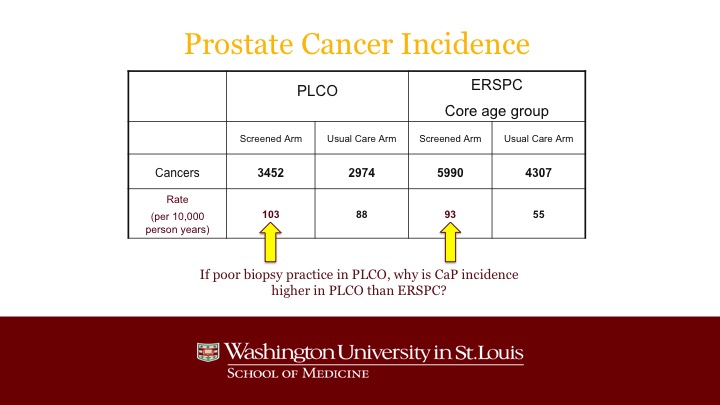
Prostate Cancer Incidence
And the reality is that, even though the population of PLCO was heavily pre-screened, and even though not every patient got a biopsy when his PSA was elevated, notwithstanding that, the actual incidence, the discovery of prostate cancer was higher than it was in the screened arm of ERSPC, and in fact you can see the main outlier was the very low detection rate in the control arm of ERSPC.
Prostate Cancer Mortality: Lower in Both arms of PLCO than either ERSPC arm
The other point to argue against that is that low biopsy rate was a problem is that the overall death rate from prostate cancer in both arms of PLCO was statistically significantly lower than the death rate of prostate cancer in the screened arm of ERSPC and certainly about half of the death rate in the control arm of ERSPC. So I do think that that criticism is not a valid criticism.
Screening and Prostate-Cancer Mortality in a Randomized European Study
Now, what about the ERSPC? This has been published twice, most recently in 2015, and I guess that publication didn’t show up.
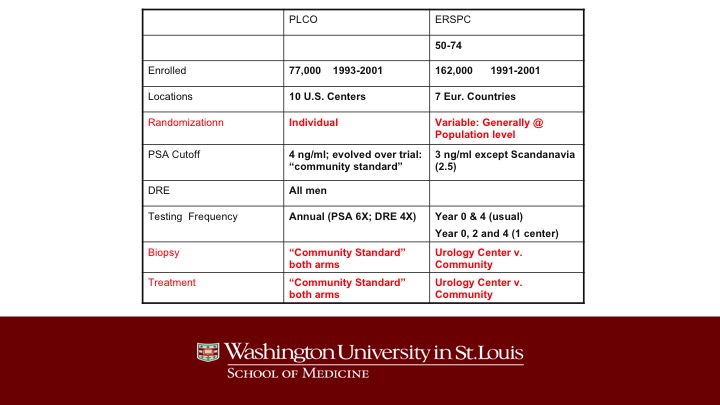
PLCO – ERSPC
Just a little bit different than PLCO. The main differences are the type of randomization in ERSPC. It was often done at the population level, and as a result of that, it turned out that if you were randomized to screening, you tended to get all of your biopsy decisions and your treatment decisions made at a urology center whereas the patients in the control arm were treated in the community, and this was totally different than in the United States where all of the care was administered in the community, not in any of the large centers.
Less Well-known Concerns: ERSPC
And so I would say there are some less well-known criticisms of PLCO. One of them was made a number of years ago by Peter Boyle and Otis Brawley that they thought the type of analysis was improper. More recently, just last year in the New England Journal of Medicine, as you know there is a debate going on whether all data from clinical trials should be uploaded onto a web page and anyone who wants can analyze that data. Some people are in favor of that. Others are opposed to that. Well, the PLCO data has been uploaded and is freely available, but the ERSPC have not yet been done so.
There is this 20% mortality was only observed in some of the countries, and it does not include men of all ages who were screened. It does not include all of the countries where screening occurred.
USPSTF
And you can see the forest plot from the original US Preventive Services Task Force showing that it was only in Sweden and the Netherlands where there was a statistically significant reduction. The big problem was Finland because Finland is the largest component of the ERSPC.
Prostate Cancer Mortality in the Finnish Randomized Screening Trial
It’s actually larger than PLCO, and it had many more events in terms of prostate cancer deaths than we did in the United States, 415 versus only 174 in the U.S. And it did not show any statistically significant reduction in prostate cancer mortality. I have some updated information on that later that I will show you.
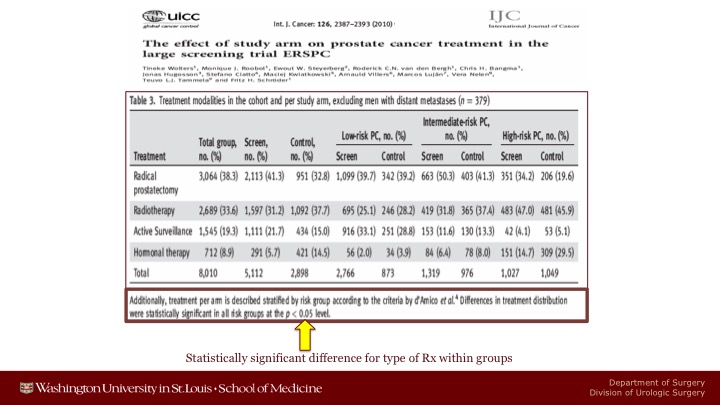
Int. J. Cancer
But I think one of the crucial problems in the ERSPC is an artifact of the fact that patients who were screened were screened were treated at major centers whereas patients who were not screened were treated in the community. And this resulted in statistically significant treatment type when you looked at low-risk, intermediate-risk, or high-risk localized disease. And you just have to look at the high-risk population. You can see I can’t read it from here about 35% of the patients in the screened arm got a radical prostatectomy versus about 20%. On the other hand, many more patients in the control arm received only hormonal therapy than were in the screened arm.
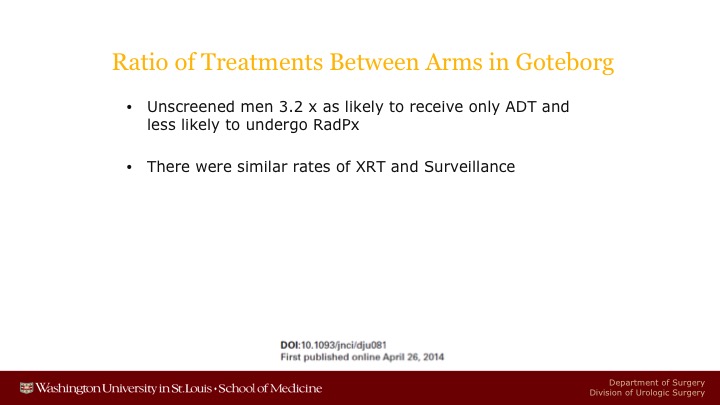
Ratio of Treatments Between Arms in Goteborg
There now have been data reported from Goteborg showing that unscreened men were 3.2 times as likely to receive only androgen deprivation therapy, and about 50% less likely to get a radical prostatectomy.
Treatment Differences in Rotterdam Arm of ERSPC
When you looked at the Rotterdam of ERSPC, you see the same thing. Control patients were more than twice as likely to get hormonal therapy. Screened patients were more than twice as likely to get a radical prostatectomy, and the treatments work. We know radical prostatectomy works.
IJC
And furthermore treatment location also differed, so screened patients were six times more likely to be biopsied and treated at a large academic center. They probably had better radiation therapy if the man got radiation. Maybe they did a better radical prostatectomy. Maybe they got better adjuvant therapy because of aggressive medical oncologists. Not all of that has been quantified.
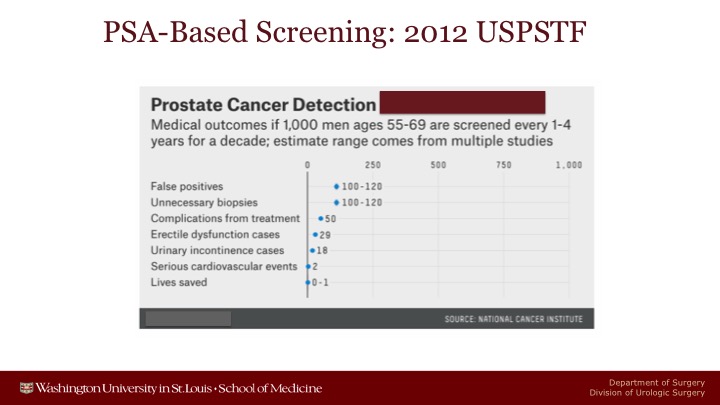
PSA-Based Screening: 2012 USPSTF
And these were the data, and we heard an exhaustive talk on the US Preventive Services Task Force and the draft.
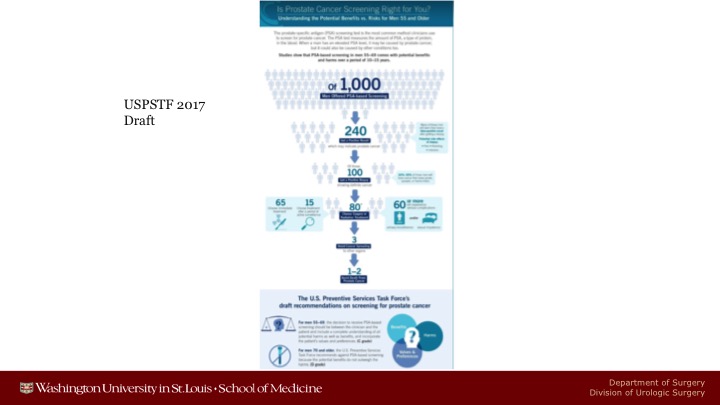
USPSTF 2017 Draft
The only difference between 2012 is that in 2012 they estimated between 0 and 1 patients life would be saved after a decade of screening.
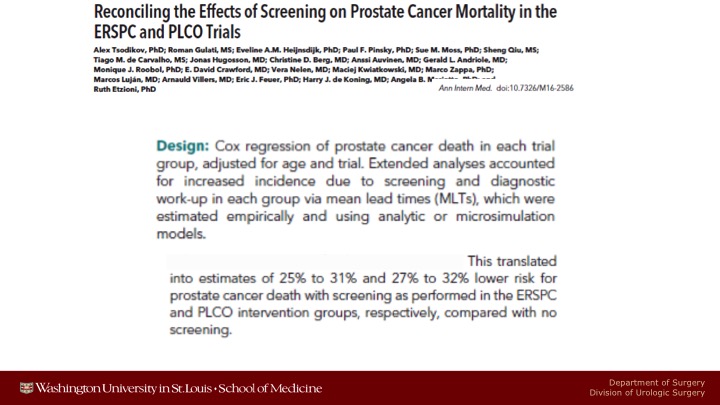
Reconciling the Effects of Screening on Prostate Cancer Mortality in the ERSPC and PLCO Trials
A few months ago, Ruth Etzioni and her group from the University of Washington, the Fred Hutchinson Cancer Center, did a re-analysis of the PLCO and some of the ERSPC data, and very sophisticated, well above my pay grade, I don’t understand everything that they did, but notwithstanding it, on the basis of what their modeling and microsimulation models that attempted to adjust for non-compliance for the men who were randomized to be screened who never got screened, for contamination in the control arm, and for behavioral patterns like that translated into net estimates that there probably is a 25 to 30% lower chance of prostate cancer death in both studies if you analyzed it in this way.
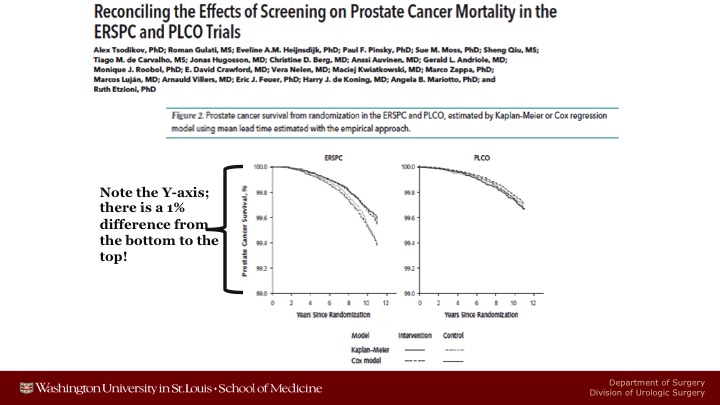
Reconciling the Effects of Screening on Prostate Cancer Mortality in the ERSPC and PLCO Trials
Here are some of the graphs that they showed. So here’s ERSPC when they do these adjusted mortality curves, you can see there is a fairly big difference between the screened arm and the control arm. The only thing I would say is that you’ve got to look carefully at the Y axis. This ranges only from a 99% chance not to die of prostate cancer to a 100% chance. So you have a lot of statistically significant difference, but it’s because there’s a large number of patients more than because the magnitude of benefit was that large. These were data just taken out to 12 years. The studies are a little bit more mature.
Detection of High Grade Prostate Cancer among PLCO Participants Using a Prespecified 4-Kallikrein Marker Panel
The reason I put that question in there is that there are now data coming out of both PLCO and ERSPC that are relevant to these issues. So here is something we published late, or about a year ago I guess it is now looking at the 4K score. Had we known about the 4K score and used it, and if we used the 7.5% cut-off that OPCO suggests for low risk, intermediate risk, and high risk, in PLCO we would have avoided 55% of the biopsies, we would have missed a few of the aggressive cancers here in the green line. The better trade-off for PLCO would have been at about a 5% cutoff because you wouldn’t have missed quite as many of the aggressive cancers.
In PLCO, we have a session going at the AUA showing that DRE still makes a difference when you look at men who develop metastatic disease in the PLCO trial, knowledge of their rectal exam status would have been helpful for that. In ERSPC, again just published last summer, they pointed out that maybe the problem with Finland, remember that is the largest component of ERSPC, very high prostate cancer death rate, did not show a statistically significant benefit. Well, maybe that is explainable by the fact that like PLCO there is a rising rate of contamination, 48% of patients at 8 years, 63% of patients at 12 years are getting contaminated and having PSA testing in the control arm.
Characteristics of Prostate Cancer Found at Fifth Screening in the European Randomized Study of Screening for Prostate Cancer Rotterdam
And finally from ERPC they looked at patients who had had multiple screens and had had at least one previous negative biopsy. And they showed that a combination of MRI and TRUS biopsy would have avoided about two-thirds of the biopsies, and the low-grade prostate cancer diagnoses in the cohort. So that’s pretty large-scale evidence that using an MRI after a negative biopsy seems to be beneficial in enhancing your ability to find aggressive cancer, and lessening your ability to discover nickel and dime Gleeson 6 cancers.
PSA Screening: Where Do We Stand?
So I would just conclude by saying if we keep doing the same things that we did in PLCO and ERSPC, screening will probably never be shown to be effective or be embraced. We just have to change what we’re doing. I think it’s fortunate that we are changing what we’re doing. These are the four things that if we really do them in a large scale will make the data of PLCO and ERSPC irrelevant because we should now screen the correct man on the basis of age. We’ve been screening too old, and family history, and their genetic predisposition. We have generally not been doing any of that. We should use reflex tests before proceeding to biopsy if you consider a man’s PSA to be abnormal. If you feel you need to do a biopsy, you should do a quality biopsy. I have changed this Alan from hand job to hand done, 12-core biopsy I think is completely sub-optimal, and that MRI or transperineal biopsies are far better. And finally, if a cancer is detected, rather than proceed directly to treatment, we need to figure out better ways, and probably these genetic tests are going to be very helpful to us. So anyway, thanks very much.
Question from Dr. Neal Shore:
Gerry, you mentioned screen age appropriate. How do you in your clinic decide? First of all, what is age appropriate? And I think we all struggle with actuarial analysis. If you’re 70 and you’re acting like you’re 50, and your parents died in their 90s, how does that—as opposed to somebody who’s 70, looks 90, and has got comorbidities? With the aging of the population, better cardiovascular health and nutrition, people are going to live longer, and as you know, the data clearly is the highest percentage of people with metastatic prostate cancer are in their early 80s. So help us. What do you do in clinic?
Dr. Andriole’s Answer:
It’s screening the proper men, and one of the sad points is that I didn’t show the data here, but if you look at men over the age of 75 who get PSA tests, and you look at their projected five-year mortality because of their comorbid conditions, about 60% of the patients who have more than a 50% chance of dying in the next five years are getting PSA tests, and that clearly is low value medicine, and that is probably what we’ve done to tarnish our own reputations. I do agree with you that there shouldn’t be a hard age limit to stop screening. It should be very personalized to that patient’s comorbidities. On the front end, I think we have to start screening men in their 40s and 50s, in their mid-40s at the latest, and I think the data—the first study came out of PLCO and unfortunately Dr. Crawford was probably the first author looking at men who start out life with very low PSAs, in PLCO, 7 years later if you started out with a PSA of less than 1, only about 2% of those patients were diagnosed with cancer. On the other hand, if your PSA was 2, 3, or 4, much higher chance that those men had developed aggressive disease. And then of course Andrew Vickers in some of the data from Europe and Hans Lilga have showed the same thing. So that’s an easy one, age adjustment. And then if we throw in family history or strong family history, as another criterion to screen patients, then I think we would do a better job screening the men who need it kind of thing.
ABOUT THE AUTHOR
Gerald L. Andriole, Jr., MD, is the global Chief Medical Officer at Prostatype Genomics. He previously was Professor and Director of Urology in the National Capital Region at the Brady Urologic Institute at Johns Hopkins University. He also formerly served as the Robert K. Royce Distinguished Professor and Chief of Urologic Surgery at Barnes-Jewish Hospital, the Siteman Cancer Center, and Washington University School of Medicine in St. Louis, Missouri.
Dr. Andriole received his medical degree from Jefferson Medical College in Philadelphia, Pennsylvania. He trained in surgery at Strong Memorial Hospital and the University of Rochester, and completed his Urology Residency at Brigham and Women’s Hospital and Harvard Medical School. Subsequently, he was a Fellow in Urologic Oncology at the National Cancer Institute in Bethesda, Maryland. Dr. Andriole has over 40 years of consistent contributions in the areas of prostate cancer screening and prevention research as well as BPH. He has contributed to over 450 peer-reviewed publications. He chaired the Prostate Committee of NCI’s PLCO Cancer Screening Trial, the Steering Committee of the international REDUCE International Prostate Cancer Prevention Trial and the Prostate Committee of the SUO Clinical Trials Consortium. He is a member of the American Urological Association, the Academy of Master Surgical Educators of the American College of Surgeons, the American Surgical Association, the American Association of Genitourinary Surgeons, and the Clinical Society of Genitourinary Surgeons, among other societies.
He has received the Outstanding Achievement Award from the Urologic Oncology Branch of NCI, the Distinguished Clinician Award from Washington University, the Alumni Award from Jefferson Medical College, and the Williams Award for Prostate Cancer Research Excellence from the AUA Urology Care Foundation, among others.