How to cite: Black, Peter. “Urine Markers for Bladder Cancer” January 27, 2018. Accessed Jan 2026. https://grandroundsinurology.com/Urine-Markers-for-bladder-cancer/
Summary:
Peter Black, MD, FRCSC, discusses the benefits and limitations of the currently FDA approved urinary biomarkers for bladder cancer. He defines criteria for the ideal biomarker, which would reduce the need for other diagnostic tests, have a high sensitivity and specificity rate, and be cost-effective for patients, as well as the possibility of using biomarkers in place of cystoscopy.
(Twitter Question) Reveal the Answer to Audience Response Question #1
- A. NMP22
- B. UroVysion
- C. Cx-Bladder Monitor
- D. AssureMDx
- E. Cytology
Answer Explanation:
Option B is the correct answer. An argument can be made for Option E, but there is a study that actually shows that B is the correct answer.
Reveal the Answer to Audience Response Question #2
- A. Urine cytology should be performed at regular intervals in every patient being monitor for non-muscle invasive disease.
- B. Cystoscopy can be safely omitted in low-risk patients if one of the new genomic urine tests is negative.
- C. According to expert opinion, NMP22 can be a useful adjunct in patients with low-risk bladder cancer under surveillance.
- D. A biomarker may be used to educate equivocal cytology.
- E. Is the CxBladder Detect can be used to screen high risk patient populations for bladder cancer?
Answer Explanation:
The answer is Option D. I think the important point is that, the other four options are in the guidelines, but they explicitly say we should not be doing those things. I think I’ll come back to that, and Sam will also be talking about the guidelines.
Urine Markers for Bladder Cancer Transcript
Click on slide to expand
The Gold Standard for Bladder Cancer Detection
So when we’re talking biomarkers, it all starts with cystoscopy. This remains our gold standard for detecting bladder cancer, whether it’s primary detection or detecting a recurrence, but we know, of course, that patients don’t like cystoscopy. We know that it’s expensive, and we know that we miss things on cystoscopy, and it’s not the perfect test.
In Search of the Ideal Bladder Cancer Biomarker
So this has driven this ongoing search for the ideal bladder cancer biomarker, ideal urine marker to enhance the performance of cystoscopy and potentially even replace some use of cystoscopy.
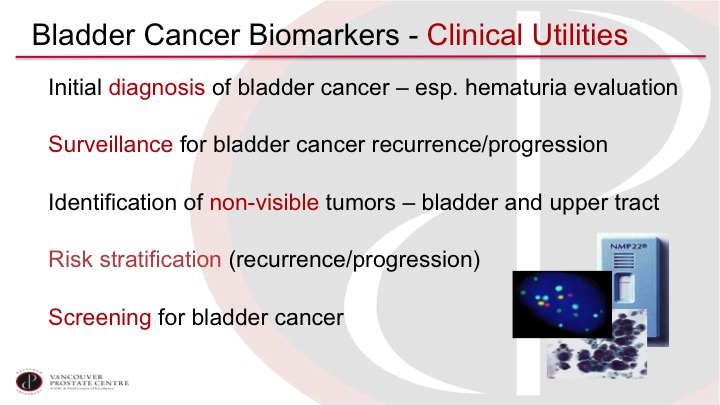
Bladder Cancer Biomarkers—Clinical Utilities
We can imagine using a biomarker for an initial diagnosis, so especially in the work-up of patients with hematuria, also for surveillance of patients, to rule out recurrence and progression after a prior non-muscle invasive bladder tumor. Of course, we would like to see tumors that we’re missing on cystoscopy so the invisible lesions, especially carcinoma in situ, but also in the upper tract lesion. It would be ideal if we could, if we could risk stratify based on markers, and of course it would be nice to be able to screen for bladder cancer with a urine marker.
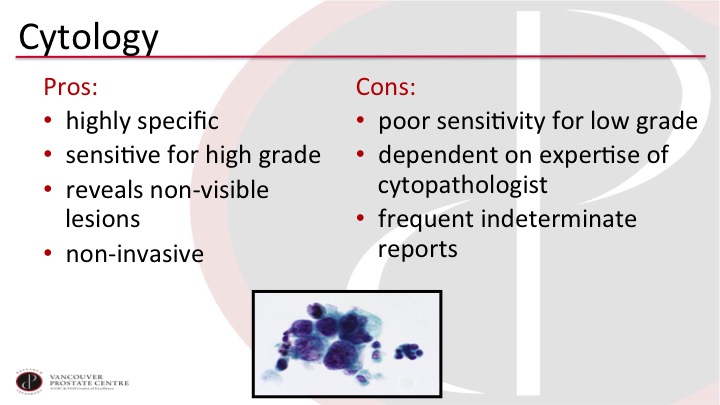
Cytology
Cytology remains the one old work-horse that I think all of us still use, or most of us probably use, and we like it because it can potentially pick up tumors that we’re missing with cystoscopy, so the non-visible ones, especially the carcinoma in situ, and we like the very high specificity of it. But we lament, of course, the poor sensitivity, and we’re always dealing with indeterminate cytology reports, which can be quite challenging to manage clinically.
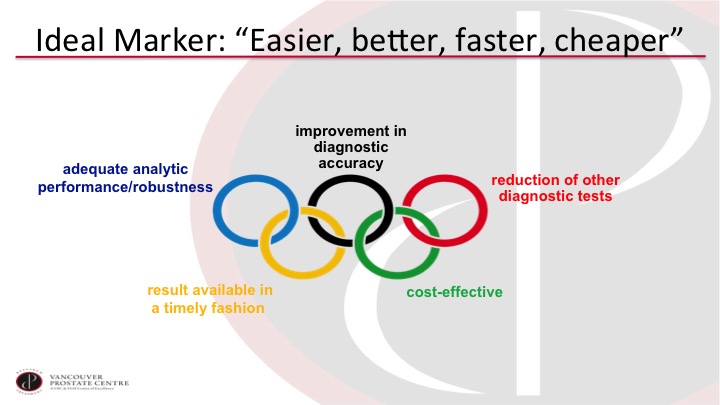
Ideal Marker: “Easier, better, faster, cheaper”
So what we really need is something that’s easier, better, faster, and cheaper. So we want a test that is analytically easy to do and has a robust pipeline. We want it to improve the accuracy of our diagnosis. Of course we want to reduce other tests, especially cystoscopy. It has to be cost-effective, and we want the report essentially while the patient is in the office or soon thereafter.
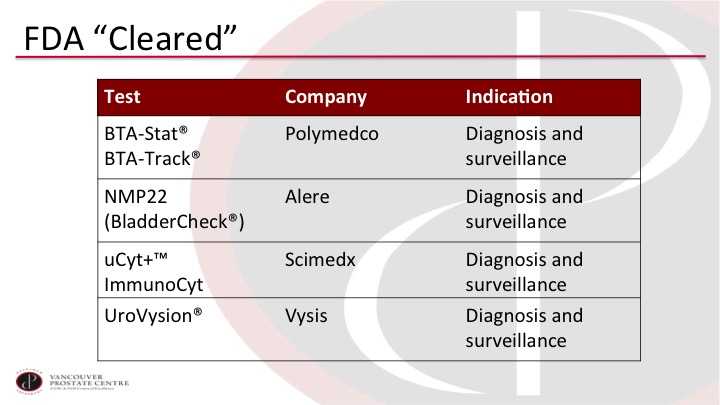
FDA “Cleared”
So we have a whole laundry list of tests, and these are just the selection of tests that have been approved or cleared by the FDA and they are all familiar to you. But none of them really meet the criteria that we would like to have for a good test.
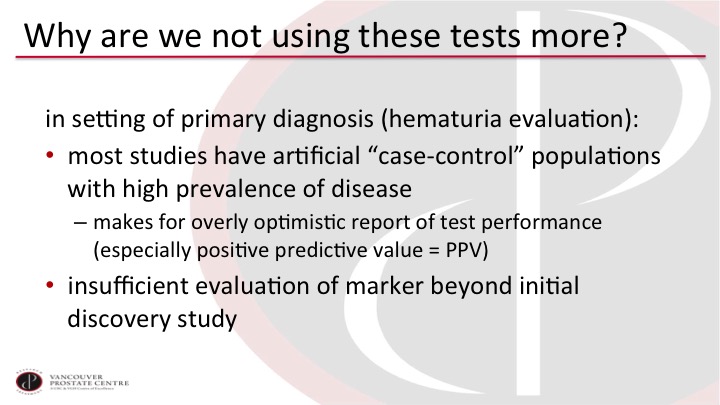
Why are we not using these tests more?
And so why are we not using these tests more? It’s always interesting to look. There’s all of these publications about so many tests. In the hematuria evaluation setting, the primary detection setting, one of the issues is that these tests are generally discovered in a case-controlled population that has a very high prevalence of disease. So if you are evaluating a patient for hematuria, only 5 to 10% of those patients in your office would have a bladder tumor. Yet in the reports in the literature for the biomarkers, it’s often 50% or so, which gives a very optimistic report of test performance that doesn’t play out in subsequent validation studies. And the biggest limitation is perhaps that we don’t usually get very many validation studies beyond the initial discovery study.
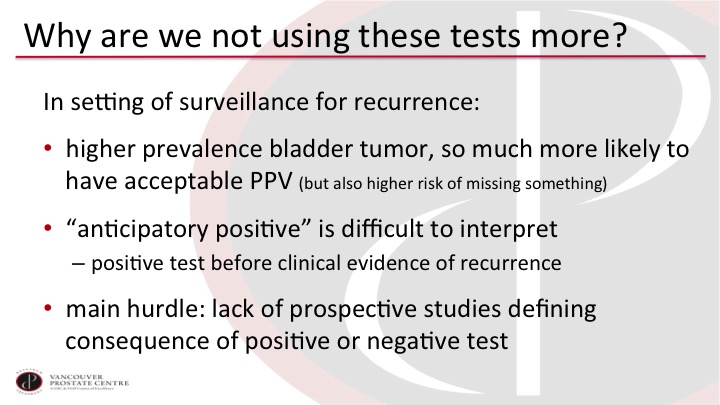
So why are we not using these tests more? #2
In the surveillance setting, it is really the same issue. Here patients are more likely to have a tumor because they have had a prior history of tumor. So you are more likely to get an acceptable positive predictive value, but you are also more likely to miss something. The anticipatory positives are difficult to interpret so positive urine marker and a negative cystoscopy may actually be something that we just don’t see yet. And most studies will not biopsy the bladder to make sure that it’s not the urine marker that is correct and the cysto that is wrong. But ultimately, the main hurdle again remains the lack of prospective validation for the biomarkers.
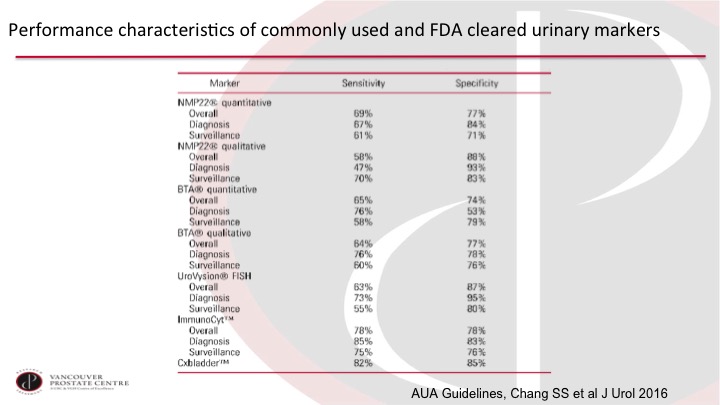
Performance characteristics of commonly used and FDA cleared urinary markers
So the AUA Guidelines Group has put together this very nice table that summarizes the overall program of the usual markers, and you can see just without honing in on any of these numbers too much that they really don’t—they are not high enough. The sensitivity and specificity are not high enough to justify routine use in clinical practice, which leads to the question: what is good enough to be used routinely in clinical practice?
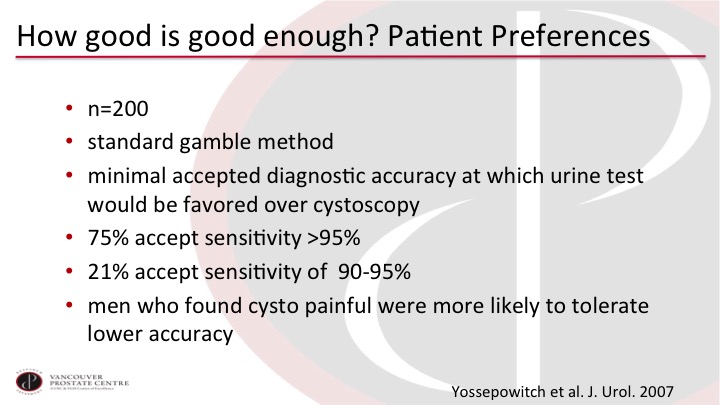
How good is good enough? Patient preferences
And the group at Memorial Sloan Kettering asked this question a few years ago. They took 200 patients and used standard gamble method to ask patients how good does a test have to be to forego cystoscopy? 75% wanted the test to have better than 95% sensitivity. 21% would accept 90 to 95. So there were almost no patients accepting less than 90%. Interestingly men who found cysto particularly unpleasant were more likely to tolerate the lower accuracy, but you can see that patients want certainly so they would rather have the cystoscopy if it provides them with more certainty.
Bladder Cancer Biomarkers – Clinical Utilities
So if we go back to these clinical utilities, most of what we are talking about is really primary detection and surveillance. I do want to hone in though on the risk stratification question because I think this is sort of a niche use of markers that has some promising studies.
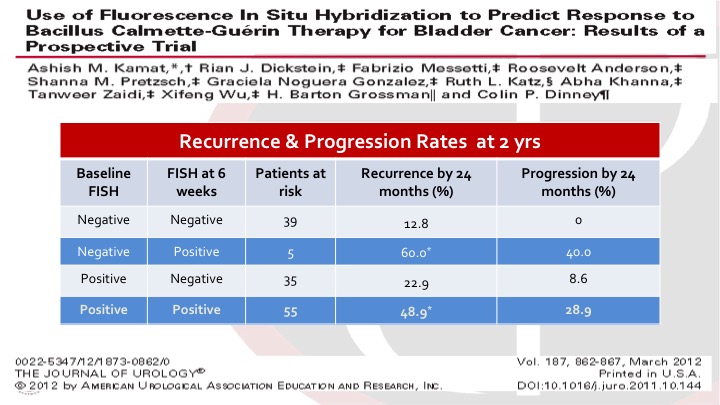
Use of Fluorescence In Situ Hybridization to Predict Response to bacillus Calmette-Guerin Therapy for Bladder Cancer: Results of a Prospective Trial
These were intermediate and high risk bladder cancer patients, non-muscle invasive bladder cancer patients getting BCG. And Ashish and the group looked at baseline FISH, so this is a UroVysion test, and also again at six weeks, I think three months, six months, additional time points as well. And it was very interesting to see that the FISH at six weeks predicted the risk of recurrence and progression. So you can see there are sort of four categories. You started negative and stayed negative. You did very well. If you started negative, and became positive, you had a very high risk of recurrence and progression, and the same if you started positive and remained positive. So this gives us an idea of how we can use these markers based on well-designed trials to guide prognostication but potentially also treatment interventions.
ACB: Practice Guidelines And Recommendations For Use of Tumor Markers in the Clinic
Nonetheless, for the bigger questions of primary detection and surveillance, the experts in the field who write the guidelines are very clear that the tests are not ready for primetime. This is a document from the Nash Academy of Clinical Biochemistry.
NMIBC AUA Guidelines 2016
And then, of course, the AUA Guidelines more recently have also laid out how we should use markers, and there are some good points here that are worth highlighting. So, in a patient who has had a prior tumor and is under surveillance, of course we do not use any of these markers to replace cysto. I don’t think any of us are tempted to do that right now. I think this is an important point, too. In patients with low-risk disease, so they’ve only had one low-grade papillary tumor, so Ta tumor previously, we actually don’t need to use any markers. We don’t need any cytology in these patients. I think a lot of urologists are probably using cytology. There’s just no need for any markers in this patient population. And where we can think of using the markers are as I alluded to in the trial that Ashish performed is looking at response to intravesical BCG and then adjudicating an equivocal cytology. So if we have an indeterminate cytology, it’s atypical or suspicious for malignancy, but not frankly malignant then a UroVysion FISH or an ImmunoCyt can be helpful.
So why are we talking about urine markers?
So if there’s so little value in urine markers, why am I even talking about them?
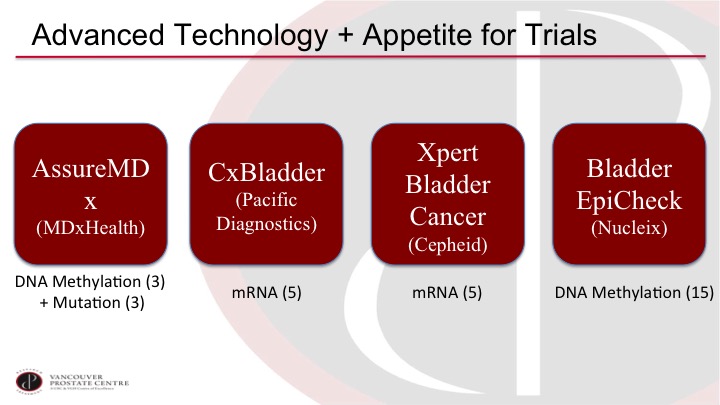
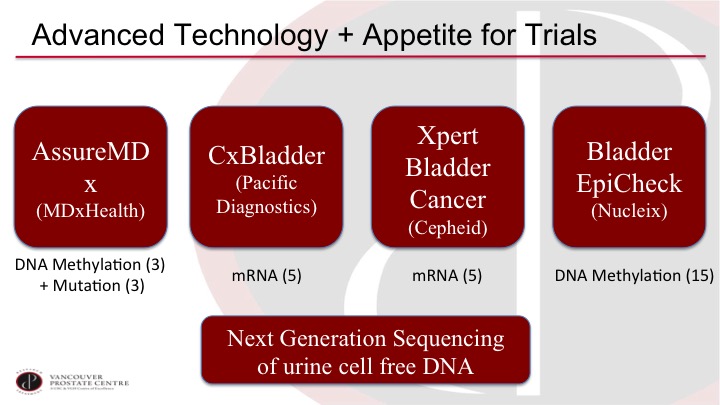
Advanced Technology + Appetite for Trials
I think it’s although I’m a bit of a marker nihilist up to now, I’m actually very optimistic on where the field is going because there are a lot of interesting developments and there seems to be a lot of interest in doing proper trials. So we have a selection of new markers on the market or close to the market and they are quite different from prior markers. So most of the prior markers are an individual marker off of a single protein, and now we’re getting into markers that are based on multiple markers, and they are mostly DNA and RNA markers, so if you look at AssureMDx for example, it is looking at three specific methylation sites, and it’s also looking at three specific mutations in combination. And then the CxBladder and the Xpert Bladder Cancer are both looking at five specific mRNAs in the urine although they are both a different selection. The five do not overlap, and then Bladder EpiCheck is based on 15 methylation markers. So these markers are quite different than the prior markers.
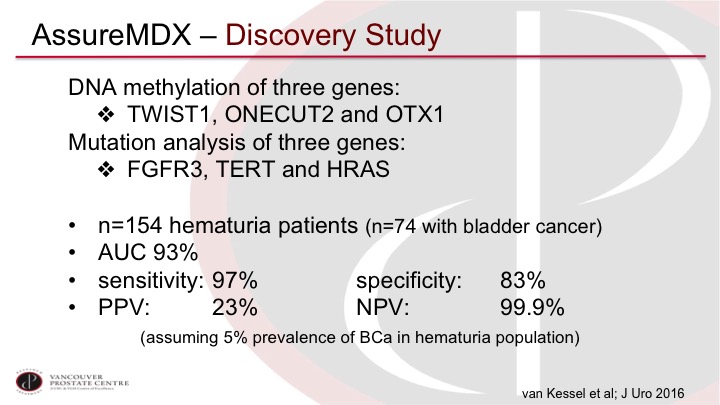
AssureMDx – Discovery Study
I’ll just go through a couple as examples. So AssureMDx was described first in this discovery study from Rotterdam, and you can see the three methylation markers there and the mutations in very common, commonly mutated genes in non-muscle invasive disease so FGFR3, TERT and HRAS. The trial is sort of your usual trial that has the main limitation that I highlighted previously, which it’s 154 hematuria patients of which approximately half have bladder cancer so that is not what we see in the real world, but exceptionally high sensitivity 97%, and a very high negative predictive value, 99% when adjusted for what a normal prevalence in the population would be. So again, this is a discovery set, and we’ve been disappointed by many discovery studies before, but it’s very promising.
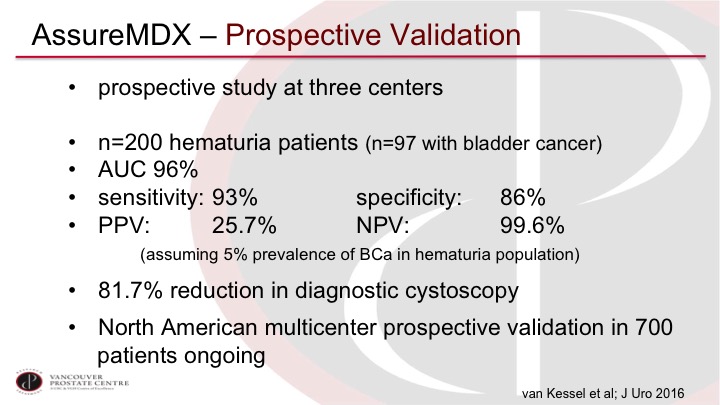
AssureMDx—Prospective Validation
Now, they subsequently went in and did a prospective validation at three centers in Europe. They had 200 patients, hematuria patients. Again, approximately half of them with bladder cancer, and very similar sensitivity in especially the negative predictive value, so these are patients where we really want to decide if they need a cystoscopy or not for evaluation of their hematuria, and so an exceptionally high negative predictive value would make us quite comfortable in potentially not doing that cystoscopy. And in this trial they calculated they could reduce potentially 81.7% of their cystoscopies. There’s an ongoing prospective validation in the US and Canada, also at our site that will be completed within a few months.
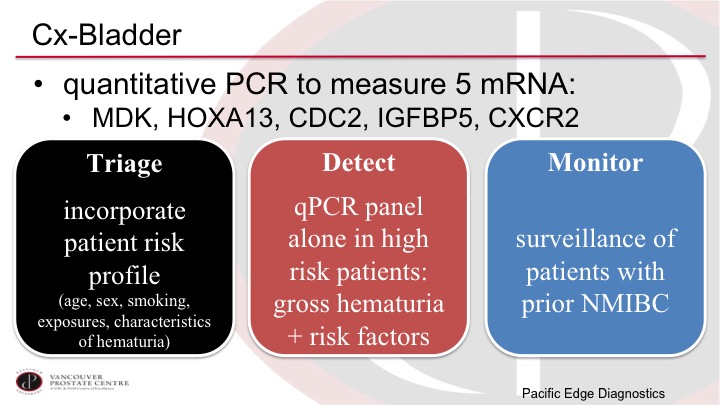
CxBladder
Another marker that is currently available in the US is the CxBladder marker. This is as I alluded to based on five different mRNAs in the urine. These markers do have potential biologic importance, they are not just random letters written up there. And the CxBladder has developed this as really three different tests, the Triage, the Detect, and the Monitor, depending on the scenario. And so the Triage is trying to select out the very low-risk patients based on clinical parameters plus the test to decide if the patient even needs a cystoscopy or not, and then the Detect is the high-risk patient with hematuria and the Monitor is the patient who has had tumors before.
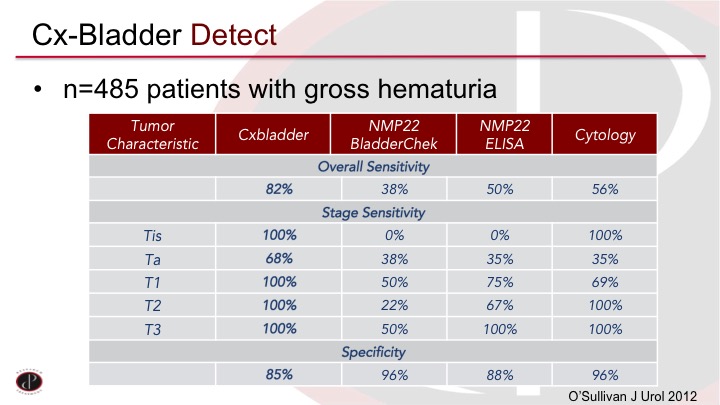
CxBladder Detect
The CxBladder Detect was published on almost 500 patients with gross hematuria compared to some of the established tests, and you can see again it’s a lot of numbers. Does this work? You can see in the first column are the CxBladder numbers so an 82% sensitivity and 85% specificity and it’s only missed a few Ta tumors in the middle there. Of course, the Ta tumors are the most common. But it did outperform the established tests other than, of course, the specificity of cytology was very high.
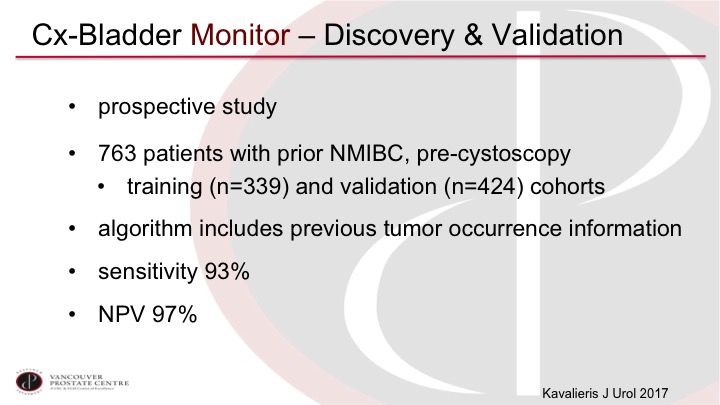
Cx-Bladder Monitor—Discovery & Validation
The CxBladder Monitor has been looked at in a prospective study of almost 800 patients that was divided into a training and a validation cohort, and again it shows very high sensitivity and negative predictive value, although again, we would need more validation to actually be used clinically.
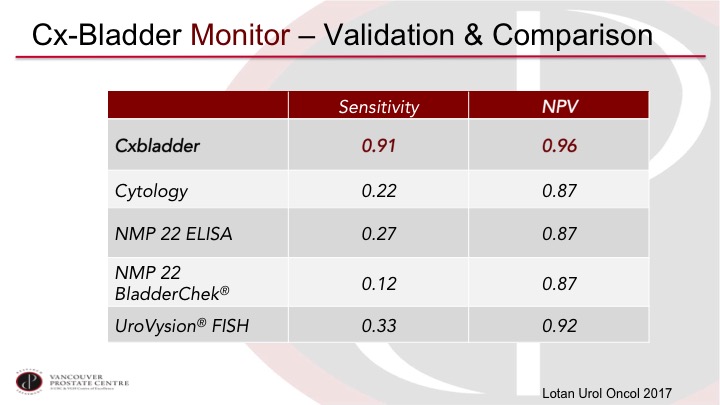
CxBladder Monitor—Validation & Comparison
Yair Lotan has published another series of 800 patients, and again, reports very high performance, so we’re getting into a performance level that I think would be much more acceptable in clinical practice.
Advanced Technology + Appetite for Trials
The path forward is also just even more exciting. I mean these are all very interesting and promising trials, but technologies continue to advance, and there is a lot of work, for example, on next generation sequencing and cell free DNA, and also digital drop PCR, exquisitely sensitive testing of specific genomic changes in the urine that you can also look at many more markers. There’s one group, for example, has a panel of over 90 markers looking at mutations and other genomic alterations that should really capture almost every patient. So I think the future is promising and we likely will be using these markers in clinical practice in the not-so-distant future.
ABOUT THE AUTHOR
Dr. Peter Black is a urologic oncologist at Vancouver General Hospital, a research scientist at the Vancouver Prostate Centre, and a Professor in the Department of Urologic Sciences at the University of British Columbia (UBC). He received his undergraduate degree from UBC and his medical degree from Johannes Gutenberg University in Mainz, Germany. He completed his urologic training at the University of Washington in Seattle and a Fellowship in Urologic Oncology at MD Anderson Cancer Center in Houston, Texas. He has a clinical subspecialty interest in bladder cancer. He maintains a grant-funded translational research program in urothelial carcinoma and is an active cancer clinical trialist in this field.