In the first part in a four-part series, Leonard G. Gomella, MD, FACS, presents “Genetics in Prostate Cancer: Identification of Inherited Prostate Cancer Risk” for the Grand Rounds in Urology audience. This educational program is supported is Myriad Genetics. Part 2 of this series is available here and Part 3 is available here.
How to cite: Gomella, Leonard G. “Genetics in Prostate Cancer: Identification of Inherited Prostate Cancer Risk” May 8, 2019. Accessed Sep 2025. https://grandroundsinurology.com/genetics-in-prostate-cancer-identification-of-inherited-prostate-cancer-risk-2/
Genetics in Prostate Cancer: Identification of Inherited Prostate Cancer Risk – Summary:
Leonard G. Gomella, MD, FACS, reviews basic concepts of genetics and genomics, the role of genetics and genomics testing in the management of prostate cancer, and methods of genomic tissue testing. He also emphasizes the difference between recreational genomics and genetic testing supervised and mentored by healthcare professionals.
The Use of Genetics in Medicine
The completion of the Human Genome Project, a thirteen year project to sequence all 3.2 billion base pairs of DNA in human cells, caused a proliferation of the use of genetics in medicine. Then, in 2013, Angelina Jolie brought the concept of inherited genetic risk of developing cancer to the public eye. Angelina Jolie’s public discussion focused on breast cancer, but the role of genetic testing is expanding into prostate cancer (PCa) treatment and management.
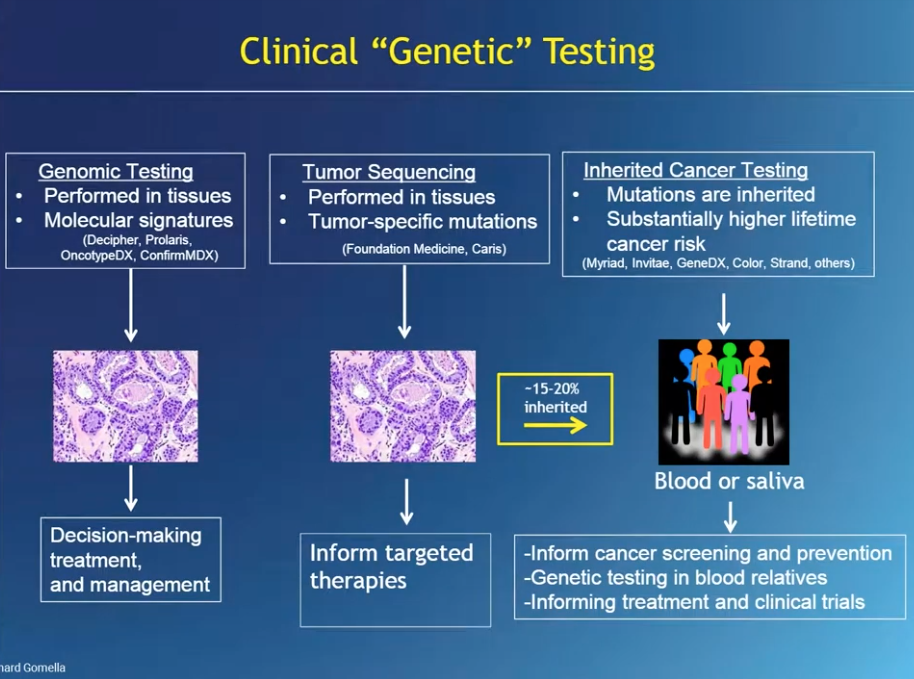
How Do Genetics Tests Work?
One option for genomic tissue testing is the Prolaris test, which looks at a specific genetic signature in the tissue, done usually through archived, fresh frozen, paraffin embedded tissue, usually in a pathology lab, and sent to a commercial lab. Results of this test give details of the cancer related to aggressiveness and prognosis.
Germline genetic testing is commonly done with saliva or blood samples. These tests identify germline mutations that may be present and will be present in every cell, not just prostate cells. Another concept in genetic testing is deep sequencing, or next generation sequencing (NGS). In NGS, each region of DNA is evaluated multiple times, which minimizes errors and provides more accurate genetic test results.
Prostate Cancer and Inherited Risk
All cancer is driven by genetic alterations, but not all cancers are inherited. In PCa, the majority (around 80%) of patients have sporadic, non-inherited PCa. However, an example of a gene associated with purely hereditary PCa risk is HOXB13.
Regarding BRCA 1/2 mutations, these genes do not inherently cause PCa. However, when PCa does develop, the presence of these genes tend to make the cancers more aggressive, more high grade, and to spread more than cancers without these particular genetic abnormalities. People with these genes also have an increased risk for cancers like breast, ovarian, pancreatic, and other cancers.
A study by Prichard et al tested men with PCa for germline mutations known to influence PCa risk. The study results showed that in men with metastatic PCa, 11.8% had germline, or inherited, mutations, compared to only 4.6% in men with localized disease. Interestingly, if someone has localized PCa and a germline mutation, these patients are genotypically more similar to metastatic castration-resistant PCa patients.
BRCA 1/2 mutations are mutations to DNA damage response (DDR) genes. Mutations to DDR genes impact the cell’s ability to undergo proper DNA damage repair, which leads to uncontrolled growth in the cell. However, it may be possible to target these DDR mutations to treat PCa with PARP inhibitors.
Genetic Information Nondiscrimination Act (GINA)
Healthcare providers involved with genetic testing should be familiar with the Genetic Information Nondiscrimination Act (GINA). GINA prevents health insurance companies and employers from discriminating against patients based on genetic test results. Unfortunately, GINA does not protect patients from discrimination in life, disability, or long-term care insurance.
Genetic Testing Implications for the Future
The 2019 National Comprehensive Cancer Network (NCCN) Guidelines focus more on susceptibility of mutated BRCA 1/2 genes than previous guidelines. For example, in the 2019 update in the guidelines on early detection of PCa, germline testing is now recommended, while it was previously a suggestion.
Genomics also connects well to precision medicine. Looking at active surveillance, men with altered genes in BRCA 1/2 or ATM are more likely to be upgraded on active surveillance. Genetic testing may be a way in the future to identify men who could stay on long-term active surveillance.
Overall, if a patient has two or more cases of PCa in close relatives under the age of 55, more than three first degree relatives with PCa, or aggressive PCa and two or more cases of breast, ovarian, and/or pancreatic cancer in close relatives, genetic testing should be considered for that patient.
ABOUT THE AUTHOR
Leonard G. Gomella, MD, FACS, is the Bernard W. Godwin, Jr. Professor of Prostate Cancer and chair of the department of urology at Sidney Kimmel Medical College of Thomas Jefferson University in Philadelphia, Pennsylvania, where he also serves as senior director for clinical affairs. Originally from New York, Dr. Gomella completed medical school and general surgery and urology training at the University of Kentucky in Lexington. After a urologic oncology fellowship in the surgery branch of the National Cancer Institute, National Institutes of Health in Bethesda, Maryland, he joined Thomas Jefferson University in 1988 and was appointed chair of the urology department in 2002. From 1998 until 2020 he was urology chair for the Radiation Therapy Oncology Group (RTOG) (now NRG Oncology) and from 2008 until 2019 he was clinical director of the Sidney Kimmel Cancer Center Network.
Dr. Gomella is involved in translational basic science and clinical research developing new diagnostic tests and treatments for prostate, bladder, and kidney cancer through the Sidney Kimmel Cancer Center where he has co-led the Biology of Prostate Cancer Program. Dr. Gomella's team was first to use molecular techniques (RT-PCR) in 1992 to detect circulating prostate cancer micrometastases, the first report of “liquid biopsy,” a discovery that led to a new field of investigation in this disease. Dr. Gomella is also recognized for developing the multidisciplinary clinic approach to prostate cancer and was an early contributor to urologic laparoscopy. He led the urology effort in the 2017 and 2019 Philadelphia Prostate Cancer International Consensus that provided the first multidisciplinary guidance on genetic testing for prostate cancer.
Dr. Gomella has given over 600 presentations nationally and internationally and written over 600 papers, chapters and monographs in urology. He has authored and edited 63 editions of 17 different books for medical students, residents, and practicing physicians, many of which have been translated into foreign languages. Dr. Gomella has consistently earned recognition for urologic oncology and prostate cancer, including a 2015 national recognition in Newsweek. In 2007, Men’s Health Magazine listed Dr. Gomella as one of the 20 top urologists in the US. Among other awards, in 2018 the Society of Urologic Oncology presented him with a “Distinguished Service Award.” In 2019, Dr. Gomella was named Enterprise Urology Vice President for Jefferson Health. Additionally, the American Urological Association (AUA) awarded him “Honorary Membership” status in 2023 in recognition for his contributions and leadership in urologic oncology. Dr. Gomella has been president of the Mid-Atlantic section of the AUA and elected to the American Association of Genitourinary Surgeons and the prestigious Clinical Society of Genitourinary Surgeons.