Minimizing Morbidity and Maximizing Outcomes with ADT
How to cite: Klotz, Laurence. “Minimizing Morbidity and Maximizing Outcomes with ADT” April 15, 2018. Accessed Jul 2025. https://grandroundsinurology.com/minimizing-morbidity-and-maximizing-outcomes-with-adt/
Minimizing Morbidity and Maximizing Outcomes with ADT – Summary:
Laurence Klotz, MD, FRCSC, discusses causes of adverse effects of androgen deprivation therapy (ADT) treatment for prostate cancer and approaches to reducing these side effects. Specifically, Dr. Klotz advises physicians about patient counseling, prescribing innocuous drugs like bisphosphonates and metformin, targeting the adrenal pathway, and utilizing intermittent ADT.
Minimizing the Adverse Effects of ADT
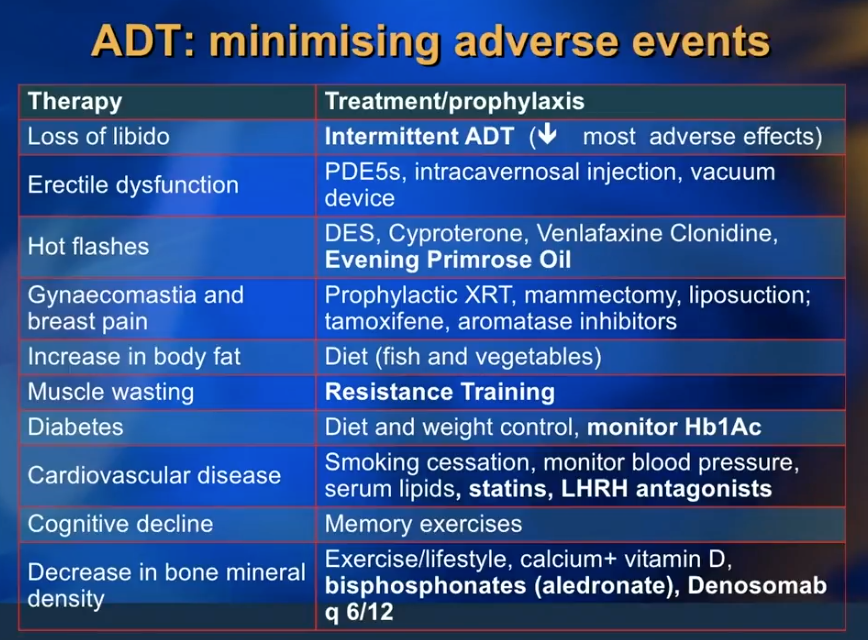
Dr. Klotz reviews practical considerations and innocuous drugs to reduce the adverse effects of ADT, such as libido loss, erectile dysfunction, hot flashes, gynecomastia and breast pain, and cognitive decline. He emphasizes counseling patients on dieting without reducing caloric intake. He then discusses the benefits of alendronate or other bisphosphonates to minimize bone mineral density loss. Metformin can reduce unwanted side effects of insulin resistance and weight gain. Additionally, he explains how exercise, particularly resistance training, can reduce ADT-related muscle wasting and delay the progression of prostate cancer.
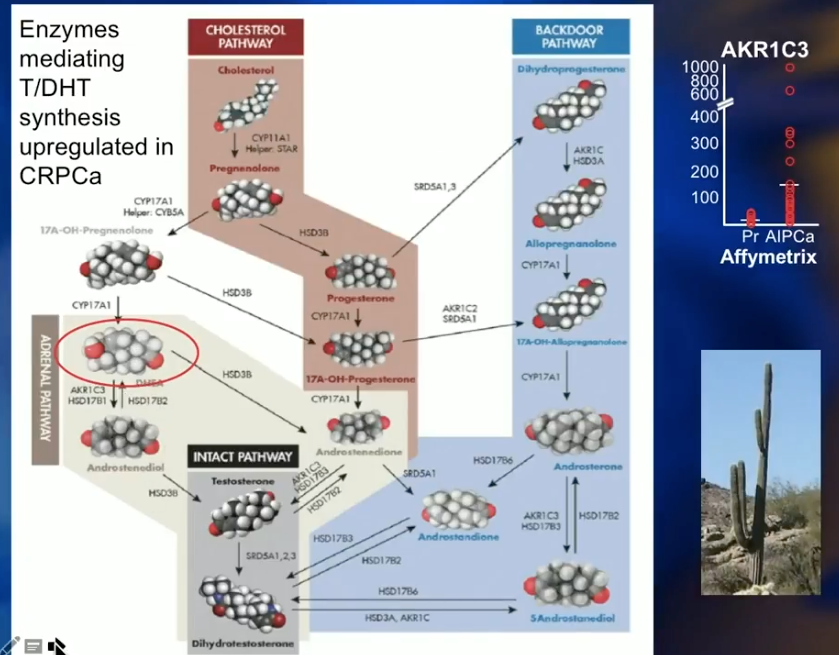
The Adrenal Pathway
The adrenal pathway involves dehydroepiandrosterone (DHEA). There is no cell surface receptor for testosterone. So, testosterone diffuses into all cells passively and equally. DHEA is a polar molecule with two hydroxyl groups and requires a SLCO2B1 transporter to enter cells. Statins also use SLCO2B1 transporters, therefore compete with DHEA to enter cells. DHEA serves as a precursor for intracellular testosterone and another route by which the cell can provide itself with testosterone and survive. However, statins inhibit the uptake of DHEA and help reduce intracellular testosterone through the adrenal pathway.
A recent study by Harshman L et al. compared prostate cancer patients receiving ADT against those receiving ADT and statins. Results showed that the addition of statins had a significant benefit in time to progression to castration-resistant prostate cancer (CRPC).
Intermittent Androgen Deprivation Therapy (IADT) and On-treatment Testosterone Levels
Although IADT is widely accepted for the non-metastatic PSA failure patient, it is still controversial for use in metastatic castration-resistant prostate cancer (mCRPC).
Dr. Klotz summarizes ten Phase III trials for IADT, each involving over 100 patients. These results consistently showed that IADT is non-inferior or had no difference compared to continuous ADT. The only exception to this observation was the controversial inconclusive results from SWOG 396.
Additionally, due to a lack of consensus on the duration of induction in IADT, Dr. Klotz led a study comparing patients who received 4 months of ADT vs those who received 10 months of ADT. The results showed no difference in time to PSA recovery, testosterone recovery, and need for re-treatment.
Testosterone Levels
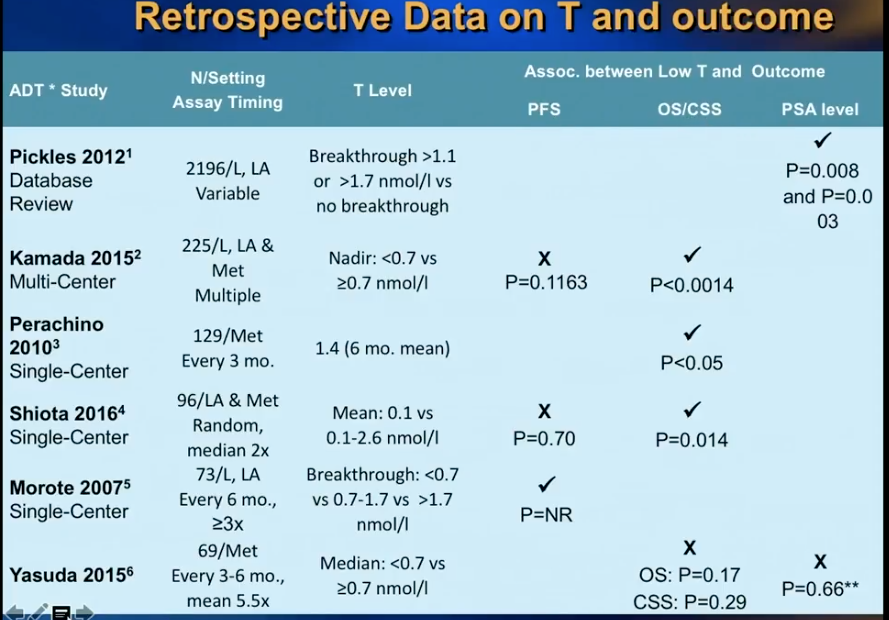
A retrospective study by Morote et al. from 2007 initiated controversy about whether or not serum testosterone levels indicate the time of progression to CRPC in patients receiving ADT. This study showed that patients who had testosterone levels of more than 50 ng/dL had 72 months of PSA progression free survival (PFS), as opposed to 90 months in patients with 20-50 ng/dL and 106 months in patients with less than 20 ng/dL.
Dr. Klotz was skeptical of the dramatic findings in this study. Therefore, he led a prospective secondary analysis of the randomized, open label PR7 trial, as that trial had collected testosterone data from patients. His analysis observed 626 patients with biochemical progression after radical therapy, treated with continuous ADT, which the PR7 meticulously followed for more than 10 years.
While Dr. Klotz thought this analysis would disprove Morote’s conclusion, in actuality, the results of the analysis vindicated Morote’s observation that that lower nadir testosterone in the first year of ADT correlates with a low time to CRPC and improved cause-specific survival.
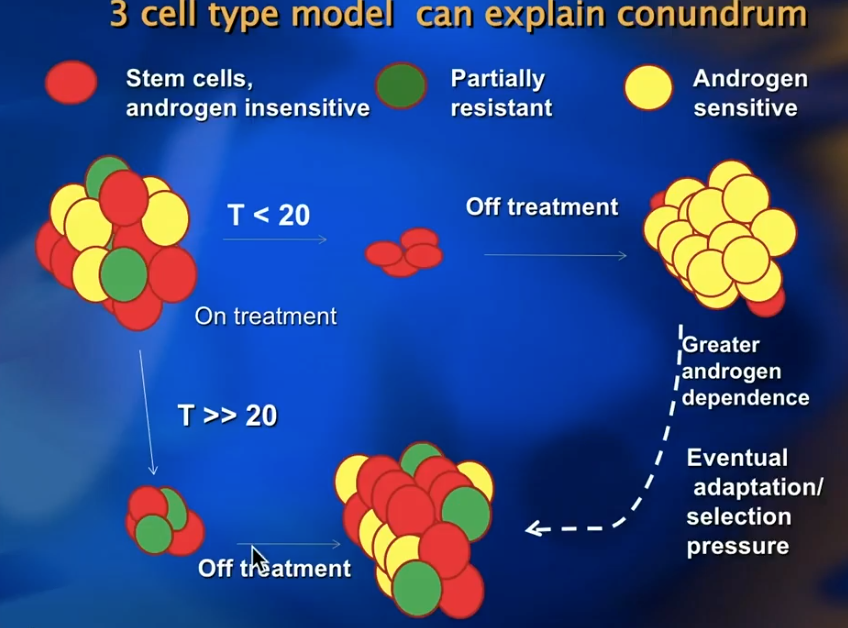
Three Cell Type Model
This concept of the best ADT outcomes resulting from the lowest testosterone levels causes a dilemma regarding intermittent ADT, since testosterone rises off-treatment.
To pose a solution to this dilemma, Dr. Klotz explains the “Three Cell Type Model.” In this model, a tumor has a heterogeneous population of cells including androgen insensitive stem cells, partially resistant cells, and androgen sensitive cells. In the first on-treatment period of IADT, clinicians should aim to lower the patient’s testosterone to less than 20 ng/dL, killing all cells other than stem cells. During off-treatment, the tumor will repopulate with a more androgen sensitive phenotype. While low testosterone levels are important, IADT can also be non-inferior to lifelong ADT.
Cardiovascular (CV) Disease: LHRH Agonists Versus Antagonists
Cardiovascular factors leads to more deaths in men with prostate cancer than the actual disease. Cardiologists define men with prostate cancer as high-risk for CV disease. The global risk estimate of myocardial infarction, stroke, or CV death in prostate cancer is over 2% per year. This global risk estimate rises to over 4% in prostate cancer patients on ADT.
Dr. Klotz was involved in a pooled analysis of 6 trials, comparing an antagonist, degarelix, to a LHRH agonist. Results showed a relatively small benefit to degarelix among all patients. In patients with baseline CV disease, degarelix showed a dramatic risk reduction compared the LHRH agonist.
The Effect of FSH Receptor Activity on CV Events
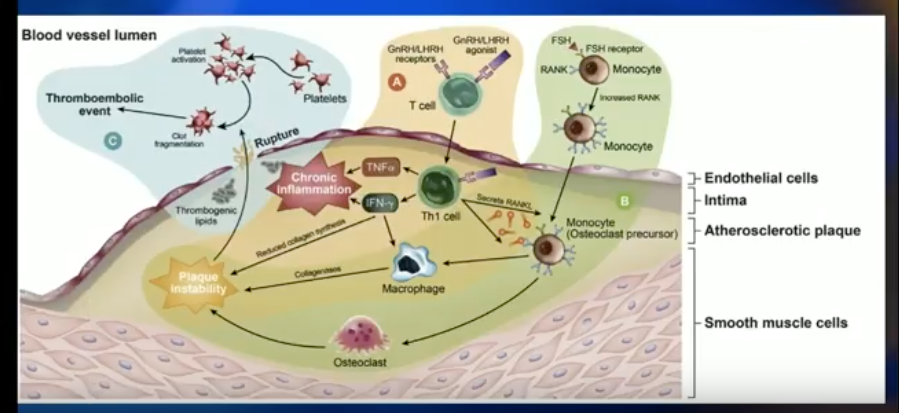
Both degarelix and LHRH agonists lower testosterone levels, inducing metabolic syndrome and puts patients at risk for insulin resistance and obesity-related CV effects. Dr. Klotz explains that there could be a difference in the CV events between patients treated with either drug due to either FSH receptor activity in prostate cancer, or the effect of LHRH agonists on inflammatory cells in endothelial plaque.
The key registration trial of degarelix showed that, in the first year of therapy, FSH levels about 45% in patients treated with LHRH agonist and about 90% in patients treated with degarelix. This is significant, because FSH is related to angiogenesis in prostate cancer tumors, tumor cell proliferation, and increased osteoclastogenesis and bone resorption. A study published in Nature observed obesogenic mice on a high-fat diet with early CV disease. Results showed that an antibody to FSH completely stopped the development of atherosclerosis and obesity in these mice.
The Effect of LHRH Agonists on CV Events
On the other hand, T cell activation by GnRH agonists may explain the difference in CV events between degarelix and the LHRH agonist. Pro-inflammatory T-helper 1 lymphocytes are macrophage activators and dominant in arterial plaques. These inflammatory cells express GnRH receptors. Also, the amount of inflammation is directly related to the degree of plaque instability. Therefore, stimulating the agonistic effect of the LHRH agonist could promote plaque destabilization.
A preclinical mouse model compared degarelix against an LHRH agonist, castration, and control. The degarelix arm had dramatically less BMI, total body weight, and necrotic plaque area than the LHRH agonist and castration arms.
Practical Considerations for ADT use in 2018
In summation, Dr. Klotz advises physicians to counsel patients on diet, exercise, and smoking cessation to reduce the adverse effect of ADT for prostate cancer. Physicians can also consider prescribing a low-dose statin or oral bisphosphonate. For non-metastatic patients and metastatic patients with excellent biochemical response, IADT is a viable option. Additionally, consider degarelix for patients with pre-existing CV conditions.
ABOUT THE AUTHOR
Laurence Klotz, MD, FRCSC, is a professor of surgery at the University of Toronto and the Sunnybrook Chair of Prostate Cancer Research. Dr. Klotz was the founding editor-in-chief of both the Canadian Journal of Urology and the Canadian Urology Association Journal (CUAJ), and he is now editor emeritus of the CUAJ. Dr. Klotz obtained his medical degree and completed his residency at the University of Toronto. He was also a uro-oncology fellow at Memorial Sloan Kettering Cancer Center in New York.
Dr. Klotz has 550 peer review publications and eight books. He coined the phrase “active surveillance” and successfully championed this approach for men with favorable-risk prostate cancer against substantial resistance. He was the associate editor of the Journal of Urology, responsible for prostate cancer, for eight years. Dr. Klotz received the Queen’s Jubilee Medal for outstanding public service, the University of Toronto's Lister Prize, the Society of Urologic Oncology’s SUO Medal, the American Urological Association’s Richard Williams Award, the University of Toronto's Lifetime Achievement Award, the Canadian Urological Association Lifetime Achievement Award, and the Harold Warwick Award from the Canadian Cancer Society for “outstanding contributions to cancer control.” In 2015 he was inducted as a Member of the Order of Canada, Canada’s highest civilian award.