Seth P. Lerner, MD, presented “Neoadjuvant/ Adjuvant Chemotherapy—Are We Ready to Accept Neoadjuvant as the Gold Standard?” at the 2nd Annual International Bladder Cancer Update on January 27, 2018 in Beaver Creek, Colorado
How to cite: Lerner, Seth P. “Neoadjuvant/Adjuvant Chemotherapy—Are We Ready to Accept Neoadjuvant as the Gold Standard?” January 27, 2018. Accessed Jun 2025. https://grandroundsinurology.com/Neoadjuvant-as-the-Gold-Standard/
Summary:
Seth P. Lerner, MD, argues for adopting neoadjuvant chemotherapy as the standard of care for metastatic bladder cancer patients. Dr. Lerner believes the urological community needs to define a better range of risk-stratification and more accurate diagnostic methods in order to do this. He discusses current developments in prognostic biomarkers and expression subtypes that he believes will make these necessary improvements possible.
Neoadjuvant/Adjuvant Chemotherapy—Are We Ready to Accept Neoadjuvant as the Gold Standard? – Transcript
Click on slide to expand
Overview
The way I’ve got the talk organized is just to provide a very brief rationale as to why we would integrate systemic therapy and definitive locoregional therapy, in this case, radical cystectomy. The evidence supporting NAC or neoadjuvant chemotherapy, the limited evidence supporting adjuvant or, post-operative treatment, our current approach, which is one size fits all, and some glimpses into precision medicine approaches where hopefully get the right treatment for the right patient.
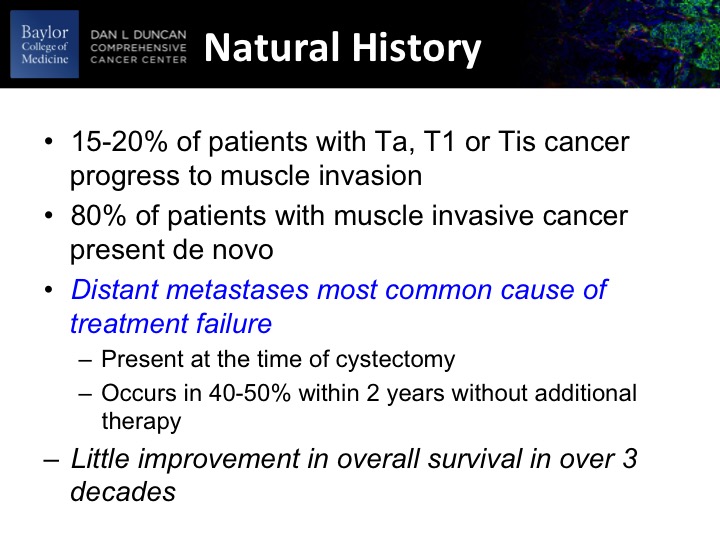
Natural History
This group is well-acquainted with natural history of urothelial cancer. Roughly about a fifth of patients with non-muscle invasive disease will ultimately progress to a muscle invasive cancer. Yet the majority of patients who are diagnosed with muscle-invasive cancer present de novo as their first experience with bladder cancer. Distant metastases are clearly the most common cause of treatment failure and subsequent death, and there’s been little improvement in overall survival probability in the last 2 to 3 decades, and that is likely to change with some of the innovations in new drug development and immunotherapy that we’ve been talking about most of the day.
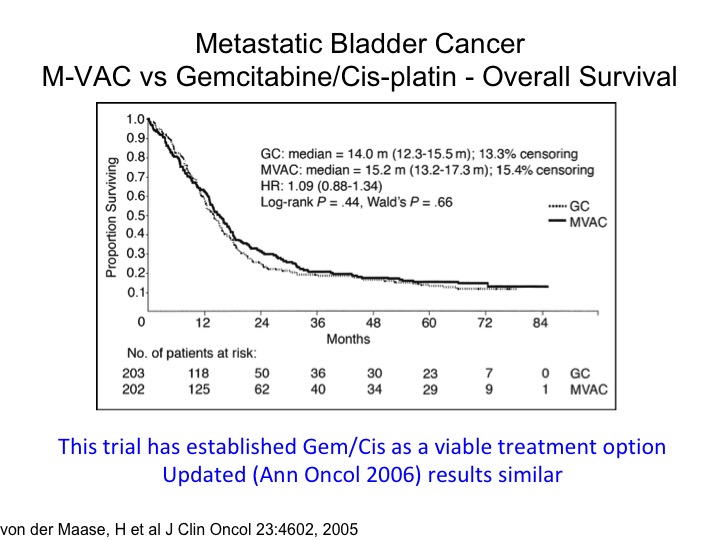
Metastatic Bladder Cancer M-VAC vs Gemcitabine/Cisplatin – Overall Survival
The tragic state of affairs. You’ll notice the publication date of 2005. This was a—you’re familiar with this, a randomized trial of M-VAC versus gemcitabine, cisplatin, and measurable metastatic disease, so the planned treatment was six cycles. It was designed as a non-inferior inferiority trial, and it shows that essentially the take-home message here is that if you looked at the two-year time point roughly about 80% of the patients have died. And you’ve got this long tail of the curve, so we’re curing a small percentage of those people, most of those patients have node only disease where you get a 30% 5-year survival probability and quite frankly not much has changed although I think the I-O era has some promise at least for a percentage of these folks. This also has been the justification for moving gemcitabine/cisplatin into the neoadjuvant or adjuvant space in the absence of level 1 evidence supporting it. I’ll go through that in a little bit down the road.
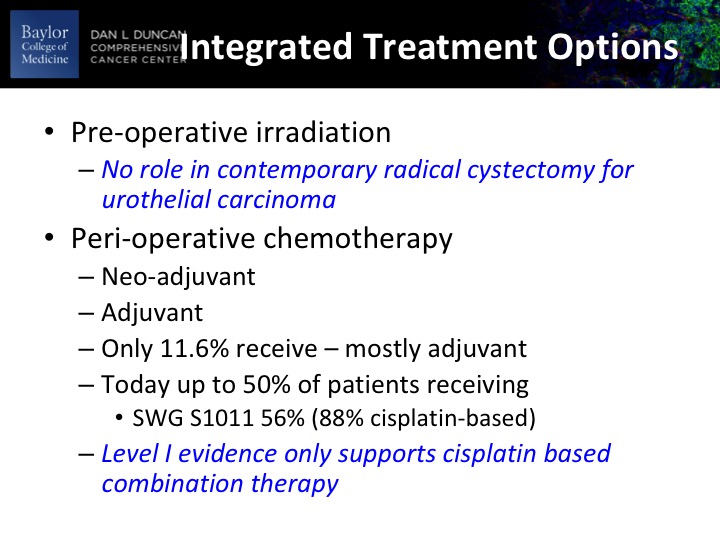
Integrated Treatment Options
So what are our integrated treatment options? There’s been a lot of work historically about using pre-operative radiation therapy. The take-home version there is that or the take-home message is that there were really no randomized clinical trials in urothelial disease that showed a benefit to preoperative radiation therapy although there may be some benefit to locally advanced pure squamous. The perioperative chemotherapy options are neoadjuvant, prior to definitive therapy or adjuvant post-operative, historically about 12% of patients received it and it was mostly adjuvant.
That needle has been shifted really over to neoadjuvant and in our S1011 randomized clinical trial of extended versus standard, no dissection. 56% of the patients got neoadjuvant chemotherapy and 88% of those were cisplatin based. Now that’s in the academic centers and probably a little slower uptake in the community, but it’s certainly on the uptake. That you heard in the guidelines talk from Sam that level 1 evidence really is only supporting Cisplatin-based neoadjuvant chemotherapy. There essentially is no role for non-cisplatin neoadjuvant chemotherapy. Remember that neoadjuvant implies no measurable metastatic disease. If you’re dealing with a patient with node-positive disease, my opinion is that they should be treated with 6 cycles of chemotherapy for metastatic disease based upon the trial that I just showed you.
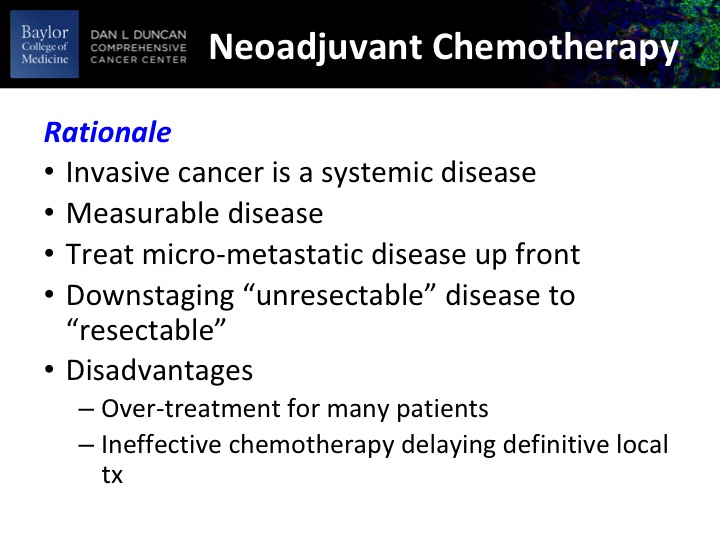
Neoadjuvant Chemotherapy
So why neoadjuvant? So the idea is that we’re dealing with a systemic disease, often with a systemic disease, and to give systemic therapy up-front before the patient has any kind of debilitating even temporary toxicities from the definitive locoregional therapy, most usual cystectomy. You do have a measurable disease in the bladder so it gives you something to get a readout on the effect of your chemotherapy. Treat the micro-metastatic disease up front. In a slightly different paradigm if you’ve got a bulky tumor or a T4B tumor, which is fixed to adjacent structures you may get or simply a big T4 tumor, you may get some down staging, and that may help the surgeon with the intraoperatively with the cystectomy. The disadvantages are fairly obvious, and you will see that on average about 40 to 50% of the patients benefit from this, which means that at least 50% of patients are getting toxicity without benefit and potentially delaying definitive locoregional therapy, and that’s really the bugaboo of one size fits all.
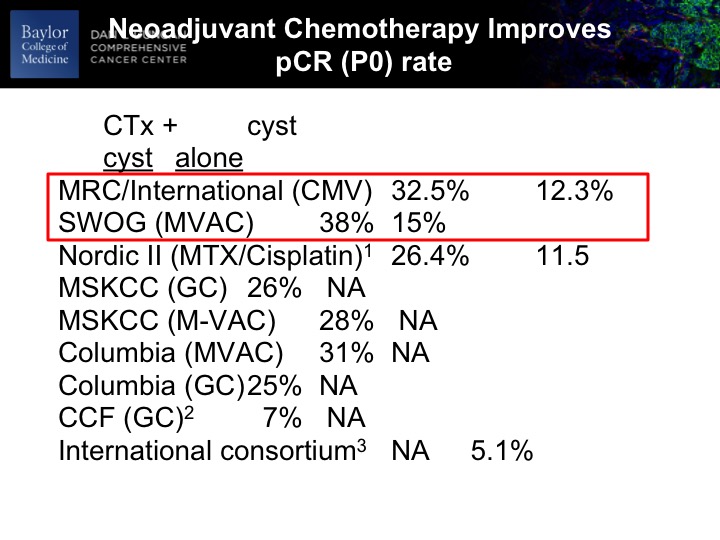
Neoadjuvant Chemotherapy Improves pCR (PO) Rate
At any rate, so if the urologists and certainly nobody in this room, but if anybody has any skepticism about whether this stuff works to the top 8900 patients. The SWOG trial roughly just shy of 400, and there was an unequivocal benefit to the chemotherapy as evidenced by PT0 rate after cystectomy. So we know that on occasion the TURBT is complete and we would expect and tell patients about 10 to 12% of the time there won’t be any residual cancer in the bladder. So we expect that. So, in the context of a randomized trial presumably balancing any kind of unknown contributing factors to this, you have nearly a tripling of the PT0 rate directly due to the chemotherapy. So it’s unequivocal. It’s undeniable. It’s there. The magnitude of that benefit in other series and some retrospective may not be on this scale, but we’re powering our clinical trials around this magnitude of minutes, so unequivocal benefit at least as measured by P0 rate, and I’m going to show you in a second that may be a reasonable surrogate biomarker for PFS and overall survival.
SWOG 8710 Neoadjuvant M-VAC Benefit cT2 vs. cT3-t4a
The other thing is that there is benefit in both clinical T2 and clinical T3-T4 disease, and this is just a stratification within the SWOG study, which shows very nicely I’ve got the median survival comparisons there, and so the current school of thought, and not every institution follows this, is everybody with a muscle invasive cancer should be counseled by a medical oncologist about the risks and benefits of neoadjuvant chemotherapy in the context of a multi-disciplinary team evaluation. When we’re dealing with definitive management for patients with muscle invasive cancer. This is a priori the very top line in ASCO guidelines, and they took the EAU guidelines and sort of made their own guidelines on that basis, and as Sam showed you before it’s front and center in the muscle invasive guidelines for the AUA. I would argue fairly forcefully that this really is the standard of care.
Now, what do you do in a patient who comes to see you, and they have an EGFR of 40 and you know that they’re not going to be able to get Cisplatin-based chemotherapy, why send them to the medical oncologist? Well, I still there is a benefit there, because there may be circumstances, let’s say they have an obstructed kidney. You put a – – or a stent in, and their kidney function may improve or their kidney function potentially could improve after surgery and it’s good to have the medical oncologist on board anyway, so just sort of how we’re trying to do things operationally at our shop.
Neoadjuvant Chemotherapy Meta Analysis 5% Survival Advantage
A meta-analysis, the magnitude of the absolute benefit is about 5%, and again it’s only accrued to multi-agent cisplatin-based chemotherapy regimens.
And this is just the KM plot from that particular meta analysis. And you could look at that and say really I’m going to treat 100% of my patients with this toxic chemotherapy for an overall benefit of 5%? And the answer to that is that is the identical magnitude of standard of care adjuvant therapy in other organ site cancers, breast colon, for example, and so we cannot ignore level 1 evidence out of convenience, and I apologize if I’m sounding like I’m lecturing, I guess, which I am. But I just want to share with you a particular viewpoint that’s shared by quite a lot of people in terms of why we should be using this.
Guidelines – AUA, EAU, ASCO
It also speaks very clearly that we have to do a better job. We have to identify the patients who are most likely to benefit from this so we don’t over treat patients who clearly are not going to get a benefit. And this is just sort of an amalgam of the guidelines from the AUA, EAU and ASCO, level 1 evidence supporting it, strong recommendation from the AUA, and then it’s interesting that the use of gemcitabine/cisplatin does not have the same level of evidence but I have to say that it’s been sort of a convenient regimen for the medical oncologists to use with the perception of less toxicity, but actually that’s only when you’re looking at five or six cycles of treatment. I would argue that the relative differences in toxicity with three or perhaps four cycles compared to MVAC is not as great and there’s still a lot of medical oncologists that are using MVAC. Suffice it to say that MVAC and GC are the most common regimens.
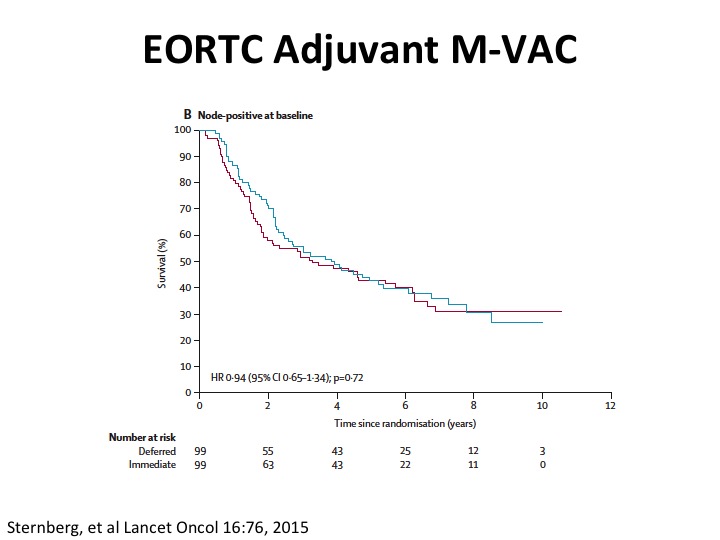
EORTC Adjuvant M-VAC
Well, what about adjuvant. So unfortunately the quality of the evidence is not so great in large part because there were three big randomized trials in Europe, Spanish-Italian and EORTC. None of them really completed accrual, and so we don’t really have good evidence. Cora Sternberg led the EORTC trial, published this paper in Lancet Oncology a couple of years ago, and just keep in mind that this is perhaps more hypothesis generating and giving us some insights because the trial did not accrue completely and was closed early because of that. The benefit at least in adjuvant appeared to be in that study from node negative rather than node positive disease.
What is interesting is you do see that the node positive patients have about this 30% five-year plus survival probability. So it’s clearly curative disease.
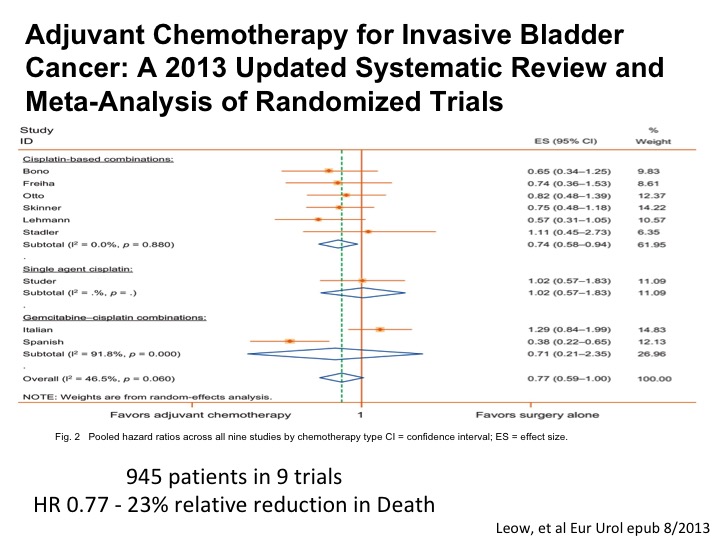
Adjuvant Chemotherapy for Invasive Bladder Cancer
An updated systematic review and meta analysis shows roughly the magnitude of benefit as we see with neoadjuvant chemotherapy. So in a setting where a patient for whatever reason doesn’t get neoadjuvant chemotherapy has high-risk disease post-cystectomy, certainly any residual muscle or perhaps T3, T4 or any positive nodes, we are recommending adjuvant chemotherapy in that setting because of the belief that the data is good enough and we want to try to do the best that we can to help the patient enjoy long-term survival
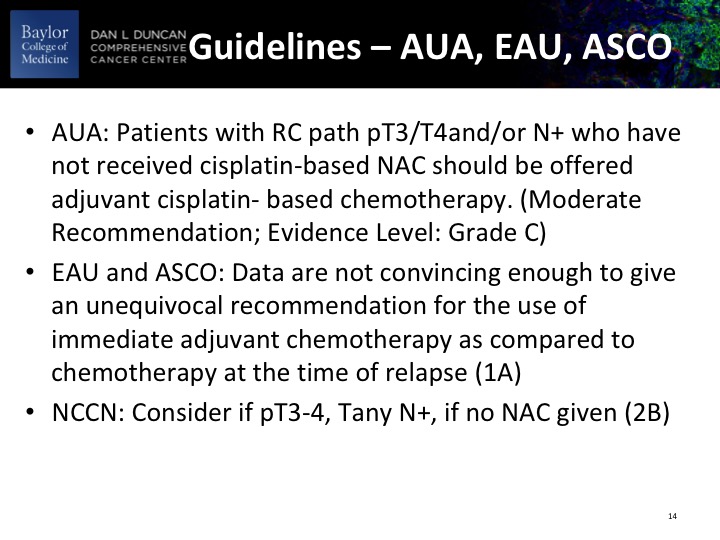
Guidelines – AUA, EAU, ASCO
These are the guidelines as they relate to adjuvant therapy, and it mostly really accrues to patients with PT3, PT4, node-positive disease.
Neoadjuvant Chemotherapy – Future
So what about the future? How do we kind of move beyond this one size fits all approach? I’ll talk a little bit about prognostic biomarkers. Those are a test that tell us something about the survival probability or the progression free survival probability and then predictive biomarkers that tell us on an individual patient basis looking at some aspect of their tumor or liquid biopsy that they are more likely to respond to the treatment of choice, so that is a predictive biomarker and then just some thoughts about precision medicine.
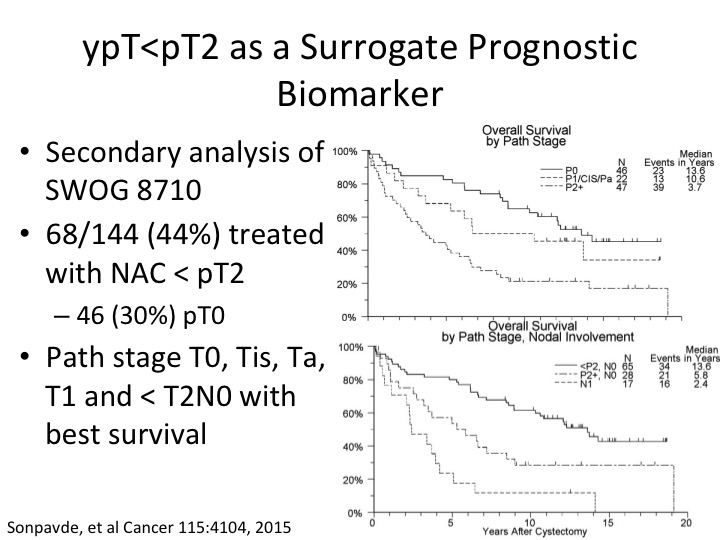
ypT<pT2 AS A Surrogate Prognostic Biomarker
What about the patient who post-chemotherapy we’re just looking at a prognostic biomarker so cisplatin-based neoadjuvant chemotherapy looking at the pathologic tumor response I showed you about PT0. So – – Sonpavde did a secondary analysis of the SWOG randomized trial and determined that with neoadjuvant chemotherapy when the pathologic tumor stage was either PT1, CIS, or PT0 about a third of those were PT0, that they had a significantly improved benefit in terms of overall survival. That, of course, doesn’t help us in a setting where we’re trying to pick patients to get neoadjuvant chemotherapy but simply just an example of perhaps an expanded definition of benefit to PT2, sorry PT<PT2 post neoadjuvant chemotherapy in that setting. And then if you look at just the patient’s—these patients have and this is just stratified I think by nodal involvement in the bottom curve.
Meta-analysis ypT0 post NAC
There’s been a recent meta analysis that was published in 2014 showing again this sort of same benefit to patients that are PT0 post neoadjuvant chemotherapy.
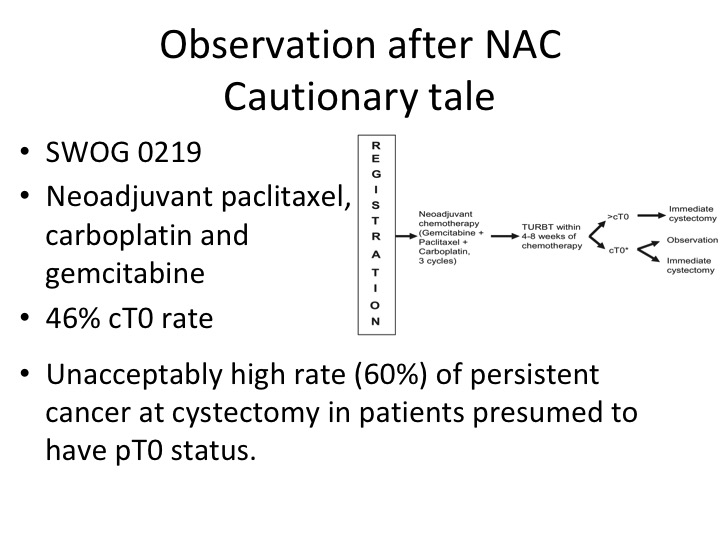
Observation after NAC Cautionary Tale
Then there is this concept that was really put forth by Cora Sternberg. She had a group of patients of about 104 patients treated with chemotherapy alone, so they had TURBT, chemotherapy, post chemotherapy TURBT and didn’t have PT0 or maybe they had carcinoma in situ, and they were observed and a significant portion of those patients enjoyed long-term survival. That is clearly not the standard of care. So I’m sure that everyone in this audience who treats these patients has had patients who come in and they say, well, doc, I have no more—you just told me that I’m cancer free after the chemotherapy. Why do I have to have my bladder out? Well, if you look at the original neoadjuvant chemotherapy data from memorial 40% of those patients, as I recall had residual disease that was CT0. Clinical T0 post neoadjuvant chemotherapy. So that was really telling us that these patients really did need to go on and have definitive surgery.
Well, we tested this in a clinical trial. This was done by Kukilar and Rafe Deveer White, SWOG 0219, David Crawford will remember this trial. It was neoadjuvant chemotherapy, non-cisplatin based, just kind of taking a stab at a different regimen and the patients who were clinical T0 had an opportunity to go on and perhaps not get a cystectomy. But what we found was that in the patients that did have a cystectomy there was a 60% residual tumor rate, and that kind of put the kibosh even though it was a small study on this idea that patients were somehow cured with cystectomy. Now, if you wait a few slides, I’m going to tell you sort of fast forward what at least part of the community is thinking about now.
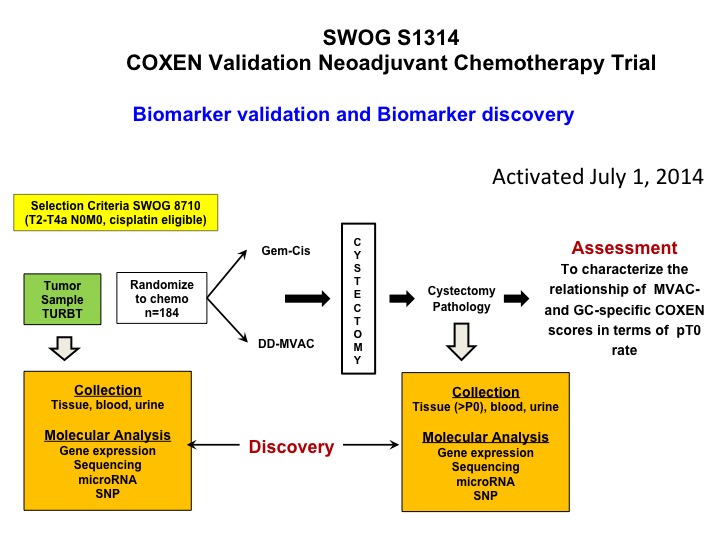
SWOG S1314 COXEN Validation Neoadjuvant Chemotherapy Trial
Now let’s get into predictive biomarkers and a nod to Dan Teodorescu and Tom Flay, who is a medical oncologist at the University of Colorado. This is a SWOG study that’s complete accrual so Dan a number of years ago developed an algorithm that he calls COXEN and it was essentially kind of a coming together of 15,000 drugs in the NCI drug repository and the NCI 60- cell line. So what he did is he did gene expression profiling on all the cell lines. By the way none of them were bladder, and looked at sensitivity, predicted sensitivity for each of these chemotherapy drugs and basically put an algorithm, a predictive algorithm together that he called COXEN. Well, that was then tested in a whole bunch of different data sets but it had never been proven prospectively to be predictive. So this trial is a validation trial of COXEN’s predictive ability to identify patients who are going to be PT0 after either dose dense M-VAC or GC. We’ve complete accrual. We’re in the midst of doing all of the gene expression profiling, and I think that these data will be available certainly within 12 months, hopefully sooner, and there’s a ton of other cool translational stuff that we’re going to be looking at in the context of this trial. So at least one approach to predictive biomarker.
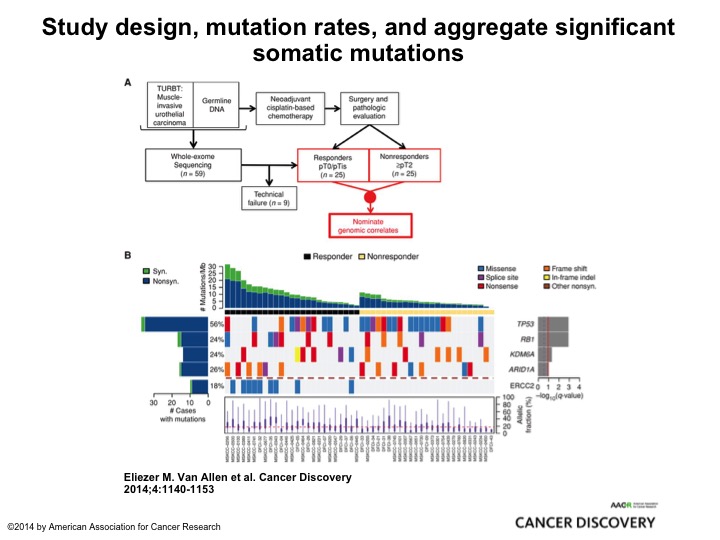
Study Design, Mutation Rates, and Aggregate Significant Somatic Mutation
This is very elegant work from Memorial by Eli Vann Allen who is a brilliant bioinformatics medical oncologist, and they took two cohorts, retrospective cohort, 25, all of them had neoadjuvant chemotherapy, 25 were PT0, 25 had residual muscle invasive cancer. They submitted it to their MSK IMPACT 400+ oncogene panel and found ERCC2 mutations associated exclusively with the PT0 response. So here was one of several looks at DNA damage repair genes and alterations identifying patients who were most likely to respond to Cisplatin and other DNA damaging agents, and there will be a clinical trial coming out of the cooperative groups from the alliance to test this strategy of identifying patients who not just with ERCC2 but about 8 or 9 different DNA damage repair genes giving them cisplatin-based chemotherapy and if they are PT0 observing them. Enriching a patient population who is highly likely to be exquisitely sensitive to cisplatin-based chemotherapy and may be able to observe its very innovative clever trial, and I think it’s really going to tell us a lot about the predictive nature of DNA damage repair and also the safety of observing these patients.
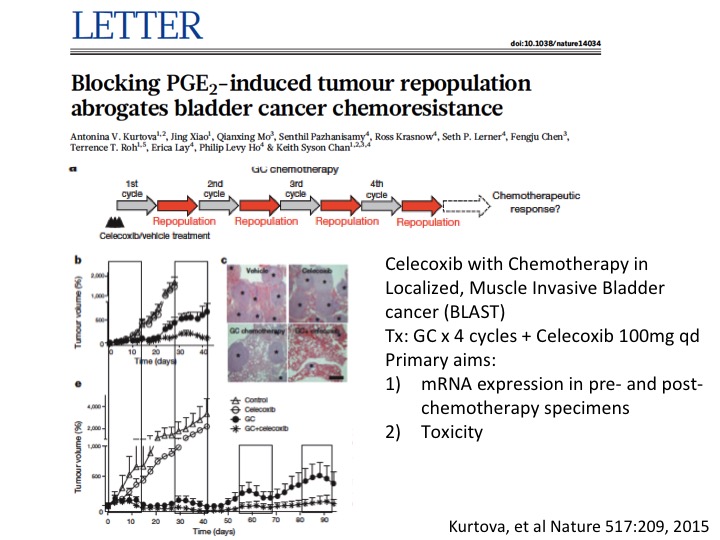
LETTER
I’ll give a little taut to my colleague Keith Chan at Baylor College of Medicine who did some very innovative work looking at other mechanisms of chemotherapy resistance and found sort of repair mechanisms mediated by prostaglandin E—by prostaglandin as mechanism for resistance and was able to overcome that with a simple COX2 blockade with Celecoxib, and that is now in a small phase 1 trial to do some validation work on this before—and if that is positive going into a larger clinical trial. So multiple sort of ways to look at mechanisms of resistance and more importantly overcoming them with a variety of interventions.
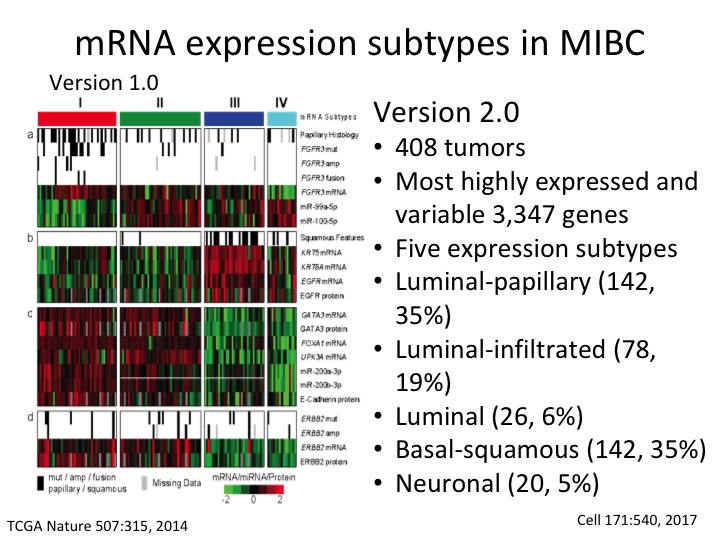
mRNA expression subtype in MIBC
This is the TCGA subtype version 1 from the Nature paper. This is the description of Version 2 where we now have five sub-types and have much more precision. We now have three luminal subtypes, luminal papillary, luminal infiltrated and luminal, and then basal squamous and a neuronal sub-type.
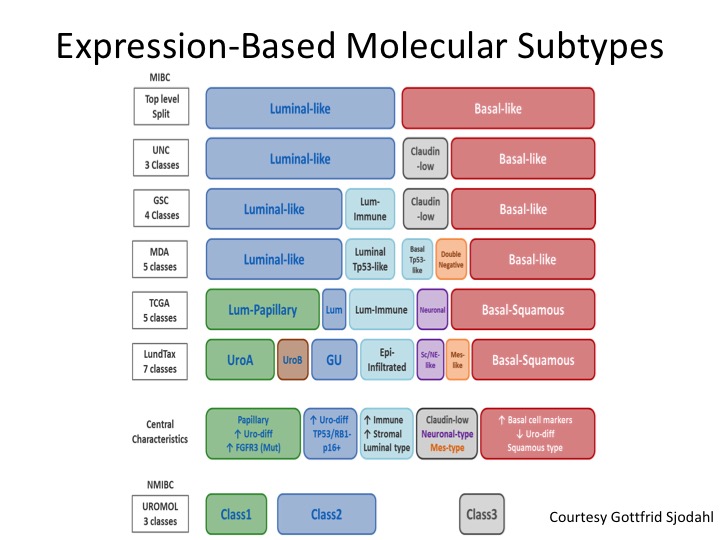
Expression-Based Molecular Subtypes
And I don’t have time to go into this, but this is a very nice diagram put forth by Gottfrid Sjodahl who is really one of the world’s leading authorities in this from the Loon Group who really started this whole thing with molecular sub-typing in muscle invasive and non-invasive disease, and you can see how the field generally has coalesced around the binary satisfaction of luminal and basal, but we’ve quickly kind of learned a lot more about differentiating within particularly the luminal sub-types and understanding the biology of those, the gene mutations and expression profiling of those and I’ll show you in a second kind of a back of the napkin approach to what the future could look like if validated from a treatment section standpoint.
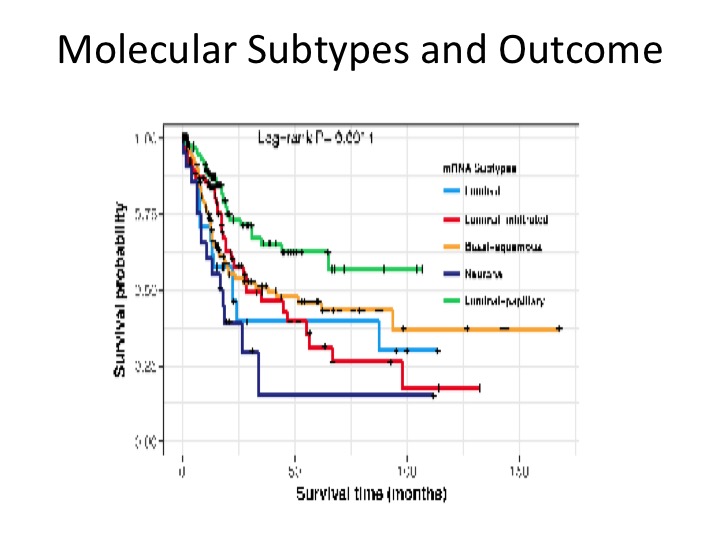
Molecular Subtypes and Outcome
These are prognostic in terms of overall survival. This is a figure from the Cell paper in November showing that the luminal papillary has the best prognosis, the neuronal has the worst with luminal infiltrate and luminal, basal, squamous, somewhere in the middle.
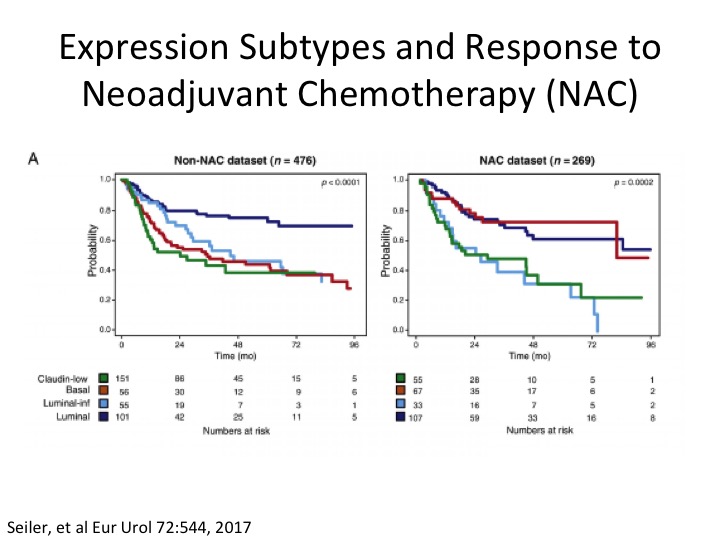
Expression Subtypes and Response to Neoadjuvant Chemotherapy (NAC)
Some clever work done by Roland Siler, Peter Black, in collaboration with GenomeDx in a neoadjuvant chemotherapy setting, where if you look on the left looking at the TCGA Version 1 subtypes, and if you notice here in red, this is the basal subtype, so non-neoadjuvant chemotherapy, and in the neoadjuvant chemotherapy dataset they were basically able to take these sort of poor prognostic basal squamous who we think are more likely to respond to chemotherapy and sure enough post neoadjuvant chemotherapy their overall survival looks more like luminal papillary, the best survival group. Another sort of piece of data in telling us a little bit about the potential predictive nature of subtypes at least in the chemotherapy sense, and you’ve already heard about the potential in an immuno-oncology setting.
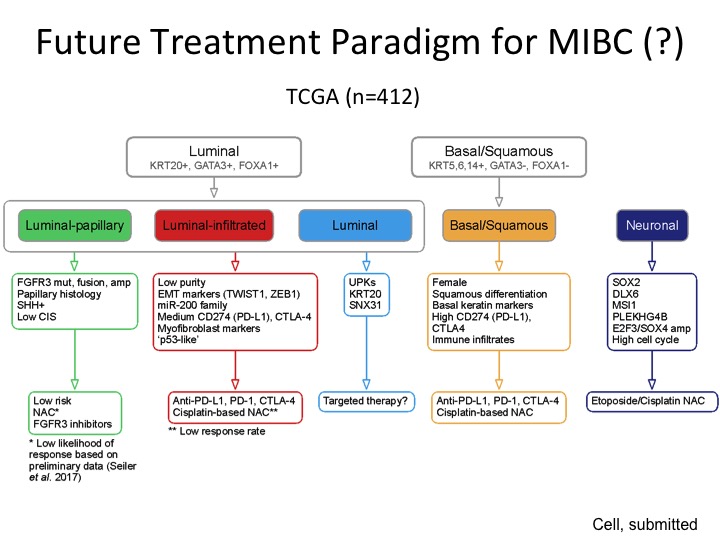
Future Treatment Paradigm for MIBC (?)
This was the back of the napkin figure, and I realize there is a lot of data here, but the way it’s organized is so we are comfortable with the binary stratification that is very consistent amongst every group that has done any kind of expression-based subtyping at least in our hands we were able to stratify this into three different groups characterized by these particular genes that are expressed, and then this is a low-risk group so we think we can observe or perhaps treat them with FGFR3 inhibitors because this is a very prominent mutation in this subgroup, luminal infiltrated by its very nature is immune infiltrated and maybe will respond better to immune-based therapy. This luminal group is a little bit more like the P53 like group from MD Anderson, a little more recalcitrant to both chemotherapy. We don’t know if they are going to respond to immunotherapy. Maybe they are better for targeted therapy, basal, squamous could respond to both chemo and immuno and the neuronal we would potentially treat like small cell neuroendocrine with those etoposide platinum, which is kind of the current regimen.
Summary
So in summary, I hope I’ve provided you at least sort of the nuts and bolts of the level 1 evidence support Cisplatin-based neoadjuvant chemotherapy. There is really not high quality evidence supporting adjuvant so our attitudes in patients who with adequate EGFR, no other sort of peripheral neuropathies that would limit them from getting Cisplatin-based chemotherapy you know we shouldn’t rely on adjuvant unless they are upstaged and didn’t get neoadjuvant. And while we’re currently in a one-size-fits-all, paradigm I think the future is going to be changing fairly rapidly as we get these clinical trials done, really do the biologic assays and get us closer to a time when the patient comes in the door, sequence their tumor, you go into this bucket, you go into this bucket, you go into this bucket, and hopefully that will also translate to better outcomes in the future ,so thank you very much.
ABOUT THE AUTHOR
Seth P. Lerner, MD, FACS, is Professor of Urology and Vice-Chair for Faculty Affairs in the Scott Department of Urology at the Baylor College of Medicine in Houston, Texas. He holds the Beth and Dave Swalm Chair in Urologic Oncology. Dr. Lerner is the Director of Urologic Oncology and the Multidisciplinary Bladder Cancer Program, also at Baylor.
Dr. Lerner earned his medical degree from the Baylor College of Medicine, completed a surgical internship at Virginia Mason Hospital in Seattle, and returned to Baylor for his residency training. He completed a two-year fellowship at the University of Southern California in Urologic Oncology and Reconstructive Surgery and joined the full-time Baylor faculty in 1992.