Sequencing of Adjuvant Therapies
How to cite: Petrylak, Daniel P.. “Sequencing of Adjuvant Therapies” April 15, 2018. Accessed [date today]. https://grandroundsinurology.com/sequencing-of-adjuvant-therapies/
Sequencing of Adjuvant Therapies – Summary:
Daniel P. Petrylak, MD, provides his perspective on optimal drug sequencing for castration resistant prostate cancer (CRPC) and metastatic CRPC (mCRPC). He reviews the literature regarding available and emerging immunotherapeutic, hormonal, cytotoxic, and DNA damaging treatment options and when to time each drug.
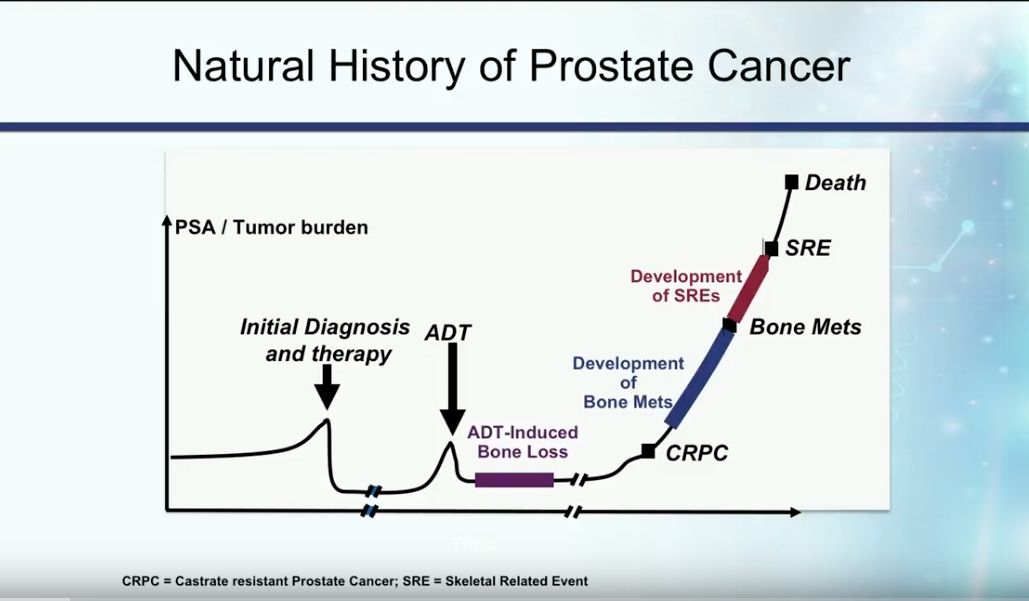
Definition and Natural History of Castration Resistant Disease
In the past, patients with metastatic disease initially presented with a high disease burden, then received androgen deprivation therapy (ADT). On average, these patients developed castration resistant disease about 18-24 months later.
Currently, the most accepted definition of castration resistant prostate cancer (CRPC) is the presence of serum testosterone levels of less than 50 ng/dL in a patient with increasing PSA or progression of the disease.
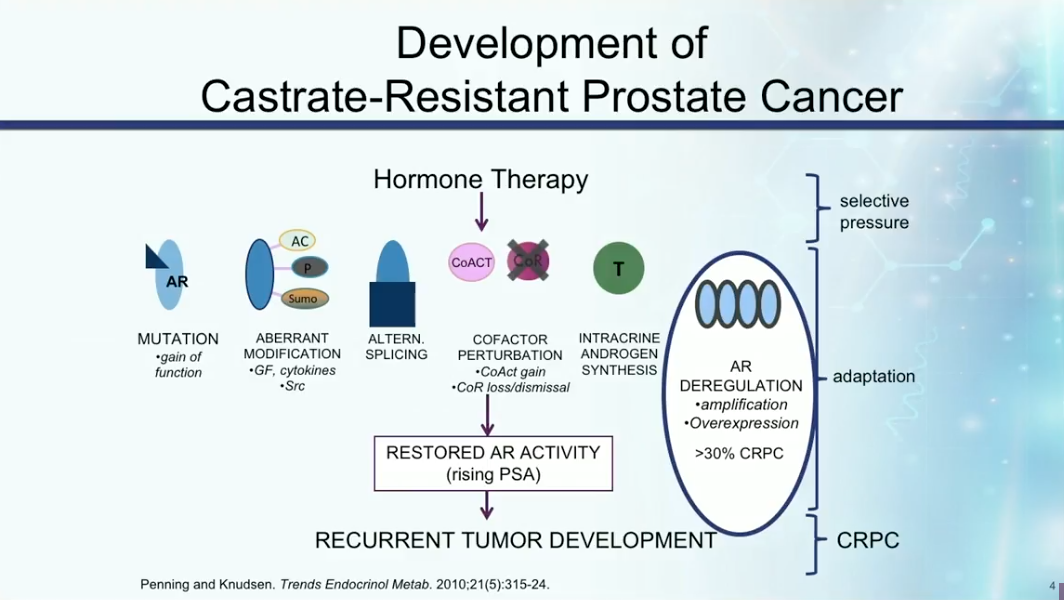
Development of CRPC
CRPC develops due to mutations in the androgen receptor (AR), aberrant modifications of the AR, intracrine androgen synthesis, or alternative splicing that cause the pathway to be constitutively active. Any of these mechanisms can cause restoration of androgen activity, rising PSA, and recurrent tumor development.
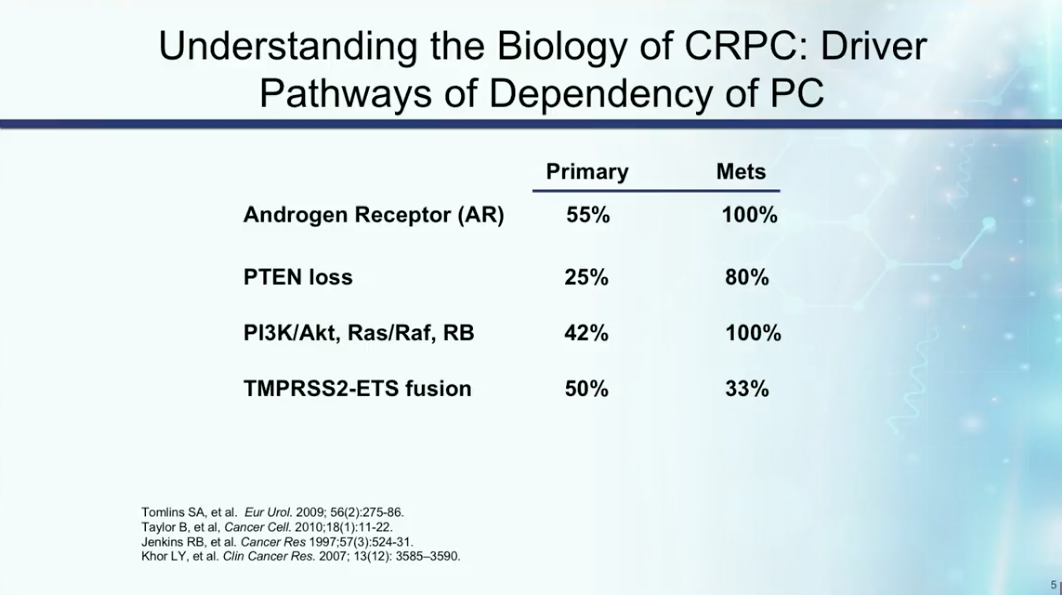
Understanding the mechanisms of castration resistance provides insights to potential drug therapies. Dr. Petrylak summarizes the rate of involvement of particular mutations and pathways involved in prostate cancer becoming castration resistant, as shown below. These mutations and pathways could therefore become targets for drug therapy in the future.
Emerging Biomarkers for Castration Resistance
AR-V7 is a splice variant of the androgen receptor associated with resistance to abiraterone and enzalutamide. AR-V7 inhibits enzalutamide’s ability to bind to the androgen receptor. However, this splice variant does not cause resistance to chemotherapy. Prospective clinical trials are currently investigating AR-V7 as a potential biomarker to help determine the best treatment options for patients.
Sequencing Agents for CRPC Treatment
The available drugs for CRPC include sipuleucel-T, with an immunotherapeutic mechanism, abiraterone, enzalutamide, and docetaxel, with hormonal mechanisms, docetaxel, with a cytotoxic mechanism, as well as radium-223 and olaparib, with DNA damaging mechanisms. All agents listed above are available at any point of the CRPC disease timeline. Additionally, the cytotoxic cabazitaxel has FDA approval for patients who have received prior docetaxel.
Dr. Petrylak believes physicians should sequencing of these agents based on the symptoms and disease characteristics of each patient. For example, he warns against using Sipuleucel-T for patients with symptomatic bone metastases. Instead, these patients should receive suggests chemotherapy, isotope therapy, or hormonal therapy.
Immunotherapy for Prostate Cancer
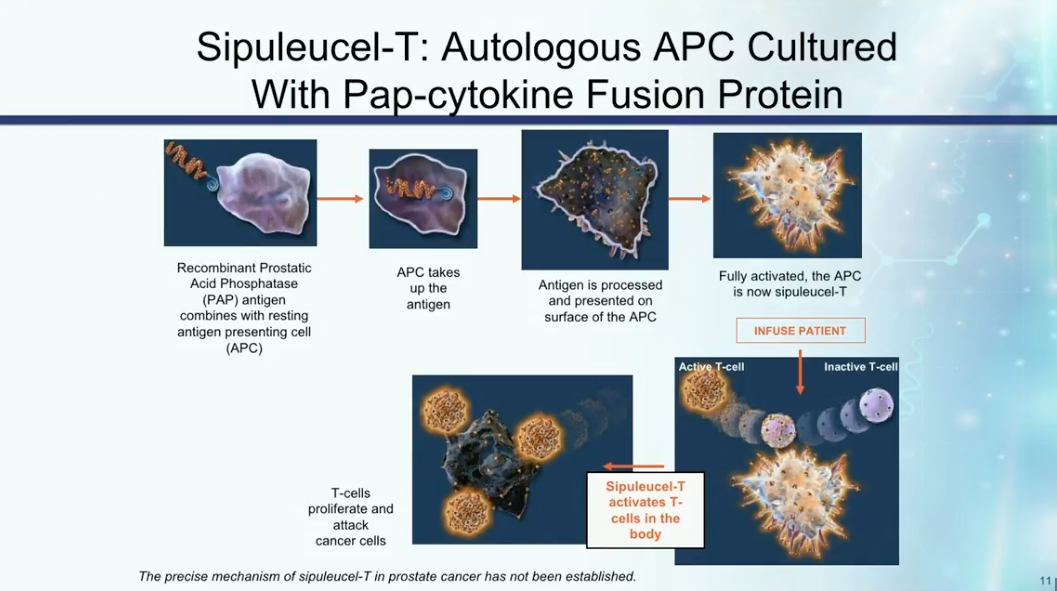
Dr. Petrylak reviews the mechanisms of action of sipuleucel-T, as illustrated in the figure to the left. He then describes three randomized trials assessing the therapy. Notably, IMPACT led to the FDA approval of sipuleucel-T and also showed that patients with lower disease burden or volume respond better to immunotherapy
Although immune checkpoint inhibitors have proven to be effective in lung and bladder cancer treatment, there is still a debate as to the efficacy of checkpoint inhibitors for prostate cancer. For example, a randomized, phase III trial by Gerritsen WR et al. investigated ipilimumab following radiotherapy. Although ipilimumab showed no difference compared to placebo in all-comers, it proved to have a survival benefit in patients with bone metastases.
There is a rationale for targeting PD-L1, as PD-L1 expresses in high levels in 52.2% of all hormone sensitive prostate cancer cases. While data does not show any benefit to nivolumab, a study by Graff NJ et al. showed activity in pembrolizumab when given to patients who have progressed on enzalutamide.
Dr. Petrylak claims that priming patients with chemotherapy and radiation to generate tumor antigen-specific T cells in order to improve the efficacy of immunotherapy in prostate cancer.
Abiraterone and mCRPC
Abiraterone acetate involves the inhibition of CYP17, as illustrated in the figure to the left. The COU-AA-301 trial assessed abiraterone following docetaxel or docetaxel plus another chemotherapy regimen. There was no difference in survival outcomes between patients who underwent either one or two prior chemotherapy. However, the absolute difference showed about a 4 month overall survival benefit to abiraterone compared to placebo.
In a study by Ryan et al. assessing abiraterone in chemo-naïve mCRPC patients, results showed that abiraterone improved radiographic progression-free survival (PFS), as well as provided an overall survival benefit.
Though these data are favorable toward abiraterone, Dr. Petrylak notes physicians need to particularly monitor elevated mineralocorticoid levels, cardiac events, hypertension, and liver function abnormalities in patients receiving this therapy.
Enzalutamide for mCRPC
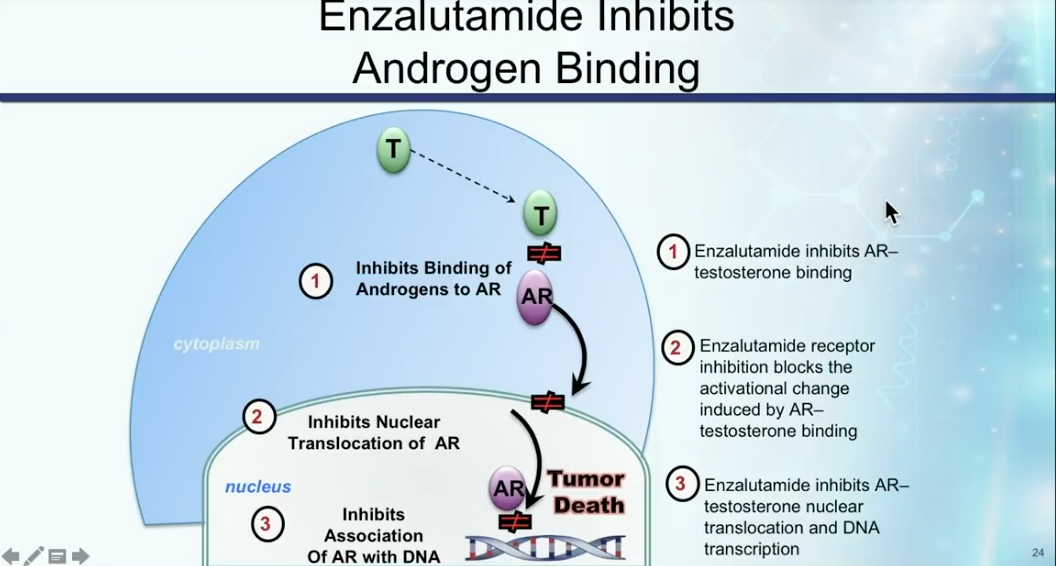
Dr. Petrylak explains the androgen receptor antagonist activity in enzalutamide, as illustrated on the left, as well as how enzalutamide differs from abiraterone. While patients must receive prednisone along with abiraterone, enzalutamide does not need this combination.
Similarly to abiraterone, data from the AFFIRM trial, which led to the FDA approval of enzalutamide in the post-chemotherapy setting, showed a 4.8 month benefit in overall survival as compared to placebo. In the PREVAIL trial assessing enzalutamide in chemo-naïve patients, enzalutamide a doubling of time to progression and a benefit to overall survival.
Unfortunately, severe fatigue is a common side effect of enzalutamide. Dr. Petrylak advises physicians to carefully dose-adjust this therapy for individual patients.
Sequencing of Abiraterone and Enzalutamide
Dr. Petrylak explains that there is not a significant amount of PSA decline, median time to progression, or median PFS related to sequencing enzalutamide and abiraterone. In other words, if a patient does not respond to one of these therapies, neither will they have a prolonged response to the other therapy.
However, Dr. Petrylak hopes to see a benefit to a combination of these therapies compares to monotherapy. A phase III study is currently investigating enzalutamide versus an enzalutamide/abiraterone/prednisone combination in chemo-naïve mCRPC patients.
Sequencing Next Generation AR-targeting Agents
Dr. Petrylak claims that the optimal timing of next generation anti-androgens seems to be earlier in prostate cancer treatment. To determine when to discontinue treatment, physicians should not only monitor PSA levels, but also radiographic progression. Physicians need to consider cross resistance when sequencing agents, specifically taking patients who are AR-V7 positive into account.
In patients with AR-V7 positive disease, Dr. Petrylak suggests considering chemotherapy. The TROPIC trial showed about a 3 month median overall survival benefit to cabazitaxel in post-docetaxel mCRPC patients. Interestingly, however, the FIRSTANA trial, which investigated treatment with cabazitaxel upfront, did not show an overall survival improvement compared to docetaxel upfront.
PARP Inhibitors
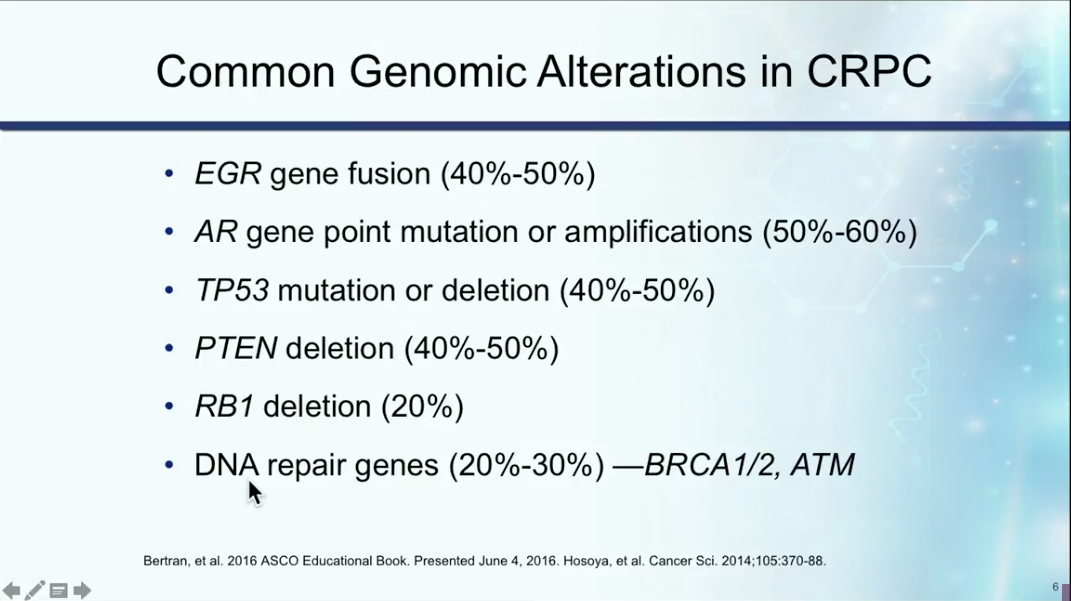
DNA repair gene alterations can impact the development of metastatic prostate cancer and occur in about 23% of castration resistant disease. Dr. Petrylak explains that the method to repair double-stranded DNA damage begins with single-stranded DNA repair. Poly-(ADP-ribose) Polymerase (PARP) affects multiple aspects of DNA repair. Therefore, PARP inhibition has durable anti-tumor activity in men with mCRPC and germline BRCA2 mutations. The five PARP inhibitors currently in phase III trials are: olaparib, rucaparib, niraparib, velaparib, and talazoparib.
The TOPARP-A study observed olaparib in men with mCRPC and found 86% of men with a DNA repair alteration responded to therapy, while only 6% of men without a DNA repair alteration had a response. Due to this trial, the FDA granted breakthrough therapy designation for olaparib for the treatment of BRCA1/2 or ATM gene-mutated mCRPC.
Conclusion
Dr. Petrylak concludes there has been rapid development and approval of agents for prostate cancer in the last 5 years. However, the rate of advancement is surpassing the understanding of managing mCRPC with these advancements. There has been much advancement in therapeutic treatments, genomic profiling, and the understanding of biomarkers and emerging targets for mCRPC. But, education gap still exist in this area.
ABOUT THE AUTHOR
Daniel P. Petrylak, MD, leads the genitourinary cancers medical oncology team at Smilow Cancer Hospital as director of the genitourinary cancer research group, professor, and co-director of the Cancer Signaling Network program. Dr. Petrylak joined Yale from Herbert Irving Cancer Center at Columbia University Medical Center with New York-Presbyterian Hospital, where he served as Professor of Medicine (Medical Oncology) and Urology and began his appointment in September of 2012. After serving for more than 20 years as the advanced bladder chair for SWOG, Dr. Petrylak is now the Vice Chair of the Genitourinary Committee.