Daniel P. Petrylak, MD, presented “Targeted Therapy for the Treatment of Advanced/Metastatic Urothelial Cancer” during the 3rd Annual International Bladder Cancer Update on January 23, 2019 in Beaver Creek, Colorado.
How to cite: Petrylak, Daniel P. “Targeted Therapy for the Treatment of Advanced/Metastatic Urothelial Cancer” January 23, 2019. Accessed Sep 2025. https://grandroundsinurology.com/targeted-therapy-for-the-treatment-of-advanced-metastatic-urothelial-cancer/
Targeted Therapy for the Treatment of Advanced/Metastatic Urothelial Cancer
Daniel P. Petrylak, MD, discusses current evidence on various pathways to target when treating metastatic urothelial cancer. He reviews agents targeting the FGFR-3 and VEGF pathways, as well as two new monoclonal antibodies, enfortumab vedotin and sacituzumab govitecan.
The Importance of Precision Medicine in Metastatic Urothelial Cancer
Investigating precision medicine for better targeted therapy in the setting of metastatic urothelial cancer (UC) is important, because only 25% of patients respond to immunotherapy, and many do not qualify for chemotherapy. However, there is no specific pathway common to all urothelial cancers. Though, there are multiple different pathways that could be exploited to treat UC. Currently, the potential targets under investigation are EGF, AKT/PI3Kinase, HER2/nue, FGR-F, and others.
FGRF-3
For example, FGFR-3 is a promising pathway to target. FGFR-3 is a growth factor pathway expressed in about 30-40% of UC specimens. FGFR-3 is a tyrosine kinase receptor and inhibition of it can cause a succession of cell growth. However, a number of mutations can cause activation of this pathway. Tyrosine kinase inhibitors (TKIs) and antibody drug conjugates are in ongoing and upcoming trials in advanced UC.
The BLC2001 study evaluated an FGFR inhibitor, erdafitinib, in heavily pretreated advanced UC patients who underwent screening for FGFR fusions or mutations. The study resulted in an impressive 40% overall response rate (ORR), and 76% experienced tumor shrinkage. The survival rate was at about 14 months, which is a promising result. There are phase III trials investigating erdafitinib further in other treatment settings.
Rationale for VEGF Blockade in Bladder Cancer
Angiogenesis, which involves the VEGF pathway, plays an important role in bladder cancer. By blocking VEGFR-2, there is synergy with chemotherapy for bladder cancer treatment. This presentation reviews clinical trial data on a gemcitabine/cisplatin and bevacizumab combination, as well as data on a ramucirumab/docetaxel combination.
Monoclonal Antibody Chemotherapy Complexes
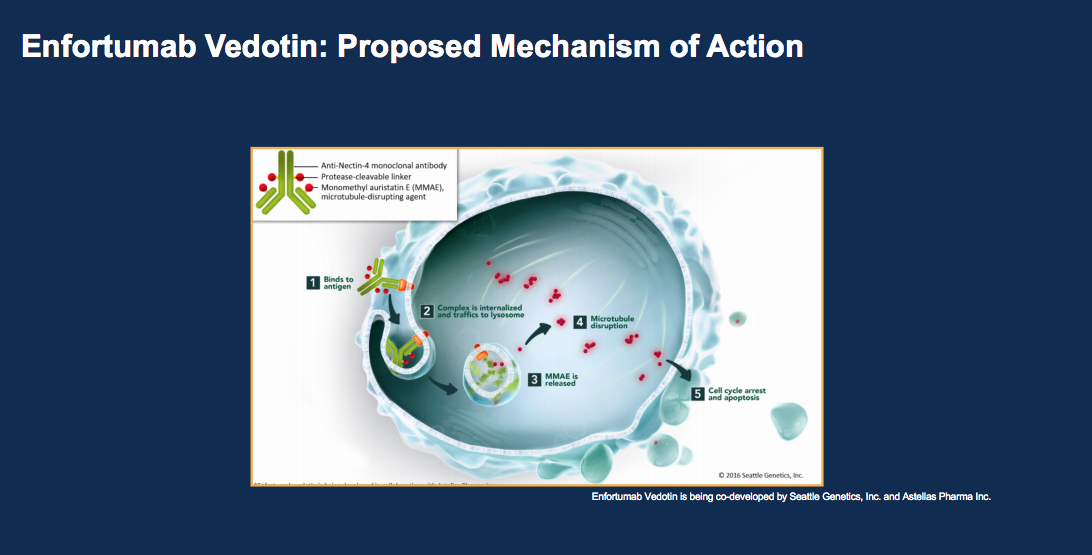
Another drug class emerging to treat bladder cancer are monoclonal antibody chemotherapy complexes. Two new drugs under investigation are enfortumab vedotin (EV) and sacituzumab govitecan (IMMU-132). EV is a complex of an antibody to Nectin, an adhesion molecule expressed on the outer portion of the urothelial carcinoma cell.
This complex is conjugated with 4 monomethyl auristatin E (MMAE) molecules per antibody, delivered to the cancer cell, internalized, and then cleaved. MMAE then has an anti-tumor effect. Administering MMAE at this level without the complex would cause more side effects, so this is an effective way to get more of the drug to cancer cells. According to UC patient samples from a Phase I trial, 97% of patients showed Nectin-4 expression. At such a high rate, there is less need to do staining as an entry criteria for EV. Currently, a Phase II trial, a Phase I trial of EV plus pembrolizumab, and a Phase III trial of EV versus chemotherapy in checkpoint failures are in progress.
Another drug in this class, sacituzumab govitecan, also appears promising. Sacituzumab govitecan is a topoisomerase inhibitor targeting Trop-2, a pan-epithelial cancer antigen.This novel antibody drug conjugate is going to Phase III studies now.
In conclusion, there are many targets evaluated for the treatment of mUC. Future research should focus on targeted therapy for the treatment of urothelial cancer and combinations with other treatments in order to improve patient response rates.
About the International Bladder Cancer Update
The International Bladder Cancer Update (IBCU) is an annual one-day CME conference focused on bladder cancer treatment updates. IBCU takes place during its sister conference, the International Prostate Cancer Update (IPCU). The conference’s faculty consists of international experts, and the event caters to urologists, urologic oncologists, and other healthcare professionals. In addition to didactic lectures, IBCU features interactive discussions, a panel roundtable, debates, and case presentations. Dr. Petrylak presented this lecture during the 3rd IBCU in 2019. Please visit this page in order to learn more about future IBCU meetings.
ABOUT THE AUTHOR
Daniel P. Petrylak, MD, leads the genitourinary cancers medical oncology team at Smilow Cancer Hospital as director of the genitourinary cancer research group, professor, and co-director of the Cancer Signaling Network program. Dr. Petrylak joined Yale from Herbert Irving Cancer Center at Columbia University Medical Center with New York-Presbyterian Hospital, where he served as Professor of Medicine (Medical Oncology) and Urology and began his appointment in September of 2012. After serving for more than 20 years as the advanced bladder chair for SWOG, Dr. Petrylak is now the Vice Chair of the Genitourinary Committee.