How to cite: Shore, Neal. “The Evolution of Understanding the USPSTF: Recommendations and Controversy” January 27, 2018. Accessed Jan 2026. https://The-Evolution-of-Understanding-the-USPSTF/
Summary:#
Neal Shore, MD, FACS, stands in for Deepak A. Kapoor, MD, at the 28th International Prostate Cancer Update, summarizing the history of the United States Preventive Services Task Force (USPSTF) and their Recommendation Grades for disease prevention and screening services. He explains the issues that arise when lawmakers attempt to use USPSTF recommendations as a basis for insurance laws, specifically in regards to how healthcare policy effects PSA testing.
The Evolution of Understanding the USPSTF: Recommendations and Controversy – Transcript#
Click on slide to expand
Reveal the Answer to Audience Response Question #1
- A. Composed of public health experts and expert stakeholders
- B. Is purely an advisory committee without payment policy authority
- C. Can choose what to review without regulatory oversight
- D. Is subject to disclosure requirements under Freedom of Information Act (FOIA)
Reveal the Answer to Audience Response Question #2
- A. Between 1966-1980, 350 modifications to the Medicare program were approved
- B. Medicare Improvements for Patients and Providers Act (MIPPA) of 2008 required coverage of USPSTF Grade “A” or “B” recommendations
- C. Balanced Budget Act of 1997 provided coverage for PSA-based screening for prostate cancer
- D. The Affordable Care Act of 2012 prohibits Medicare coverage of any preventive services with USPSTF Grade “D” recommendation
Reveal the Answer to Audience Response Question #3
- A. The USPSTF initial recommendation on PSA-based screening was its 2008 Grade “D” for men 75 and older
- B. Has yet to finalize its 2017 draft proposal
- C. Finalized its proposed 2011 Grade “D” recommendation to a Grade “C” recommendation
- D. Medicare has not proposed payment changes based on USPSTF recommendations
Reveal the Answer to Audience Response Question #4
- A. CMS proposed a policy penalizing physicians for ordering screening PSAs
- B. There has been no change in number of patients screened
- C. There has been an observed shift to lower stage/grade disease in patients newly diagnosed with prostate cancer
- D. Primary care societies have declined to endorse this recommendation
Answer Explanation:
In fact, CMS did at one point actually propose not paying, and then they very quietly withdrew it thanks to the backlash.
Reveal the Answer to Audience Response Question #5
- A. Is the only Federal advisory committee exempt from FACA
- B. Has no requirements to consider impact on stakeholders or patients of its proposals
- C. Operates essentially without Congressional oversight
- D. All of the above
Medicare—History of Preventive Services#
Interestingly, Medicare passed in 1965 during Johnson’s administration. Signed into law in 1966. Only covered diagnostic and treatment services. No coverage of primary or secondary preventive services, which is really quite a remarkable thing that we have evolved over. CMS didn’t have the authority to authorize these services at the time, and any changes in the Medicare bill at that time required congressional statutory action. By 1980 there were 350 bills covering preventive services that had introduced, and they all failed to pass.
USPSTF—Origins#
The USPSTF or United States Preventive Services Task Force was created in 1984. The role was to create recommendations regarding clinical preventive services. It took five years before they were able to publish their findings, and then there was a second task force that was empaneled, and it took another five years, or six years, to publish those findings. Ultimately, the program was transferred to another acronym governmental agency called the Agency for Healthcare Research and Quality, and ultimately, the current composition was codified in 2001. So things don’t always move very abruptly.
USPSTF—Origins Continued#
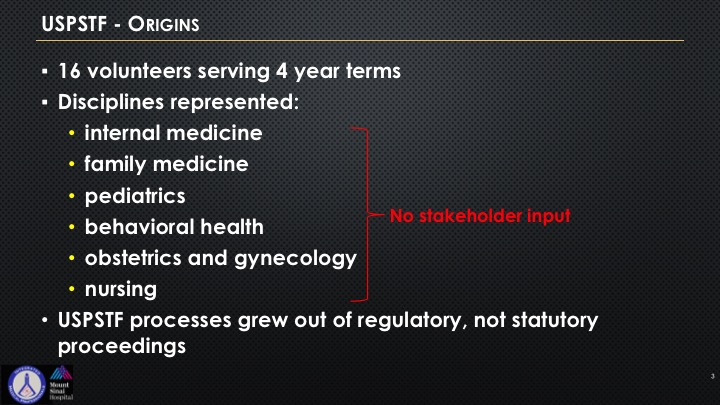
Here’s what the origins were, 16 volunteers, four-year terms, and you see these six entities that are represented. There is no mention whatsoever when you are looking at a specific disease state that a stakeholder of that specialty has to be there. So these processes grew out of regulatory but not out of statutory proceedings, which is very important because that’s one of the things that is quite unique of all of the government is that there is usually some sort of a vote. These all came from regulatory and not statutory, and so one of the biggest challenges and what the pushback has been when it relates to urology is the lack of stakeholder representation.
USPSTF—Initial Objectives#
So it had three objectives: evaluate the benefits and risks of individual screening, diagnostic services, issue recommendations about preventative services that could be incorporated into primary care, identify a research agenda. And it was initially purely advisory, and it was left to us and patients to evaluate these recommendations and decide how to best incorporate them. It was just sort of out there as one of these committees with recommendations.
USPSTF—Recommendation Grades#
And you’re familiar with these recommendations. You can get an A, a B, and I won’t go through reading it to you, but you recognize what we are currently involved with now is the D recommendations saying they’re actually against the service, and this is all germane as it relates to PSA. I is where they can’t really assess, and C is sort of a toss up. A and B is a rather positive recommendation.
USPSTF—Expanding Authority#
So the Medicare Improvements, or the MIPPA, in 2008 was established for national coverage determination processes. It removes decision making from Congress, and so that you can have this NCD process run by Medicare, but it’s influenced by—it’s “influenced” the buzz word there, by USPSTF. It allows Medicare to independently add preventive services based upon the USPSTF recommendations. The PPACA, which is part of the ACA, essentially is the ACA, the Affordable Care Act, or Obamacare, converted the discretionary role into a mandate, and also Medicare was required to recover services that were A or B. It allowed the secretary to deny any – the secretary of HHS to deny any coverages with a D rating. But the other thing interestingly is if the USPSTF decides not to rule or not to even review it, it sort of acts like a pocket veto, as you are probably familiar with from history, and it also allows the secretary of HHS to just say well, never reviewed, never ruled, we won’t cover it.
USPSTF—Issues#
Processes can be very insular, and hidden, and secretive, and very dissimilar to other transparent organizations within the government. So this notion of FACA, the Federal Advisory Committee Act, proceedings are not required to be made public, and the USPSTF has been expect from that. And this is quite unique in all of government regulatory agencies. Even most of TSA and NSA, most of that has FACA requirements. So the exemption of these administrative procedures act requires public disclosure. You see this is really quite a remarkable thing that has put us now with this maelstrom of reactivity because we were never part of this decision making. So it’s the only federal agency with the authority to set payment policy that is exempt from these two acts.
USPSTF—PCa Recommendations#
The USPSTF has issued five recommendations on screening for cancer. Started back in 2002. It was an “I” with a lack of basic consensus and resources. In 2008, they gave a “D” for men over 75. They had planned to issue a full recommendation. There was massive backlash over this, and this all sort of occurred at the time when the mammogram lobbying efforts came in and said because they really pushed back on the mammogram recommendations of the task force, and those were all put to sunder. We don’t have anywhere near that sort of media clout. In 2011, they issued a proposal “D” for PSA screening regardless of age and risk factors, and then in 2012 a “D” recommendation for PSA-based screening came out. In 2017, there was a proposal for a “C” on the 55 to 69 and again a “D” for everybody greater than age 70.
USPSTF—2012 Recommendation#
In 2012, this was finalized despite the overwhelming objections of multiple organizations I think SUO, LUGPA, AUA, AACU, and it really was that they stated no asymptomatic man should have a PSA test performed. This was this kind of crazy one-size-fits-all recommendation not looking at anything regarding any genetic, familial, hereditary prostate cancer risks, race, predispositions, African American, African Caribbean, a black Latino, prior toxin exposures, etc., and so there was a collective organized urology opposition to this.
USPSTF—Impact of Recommendation#
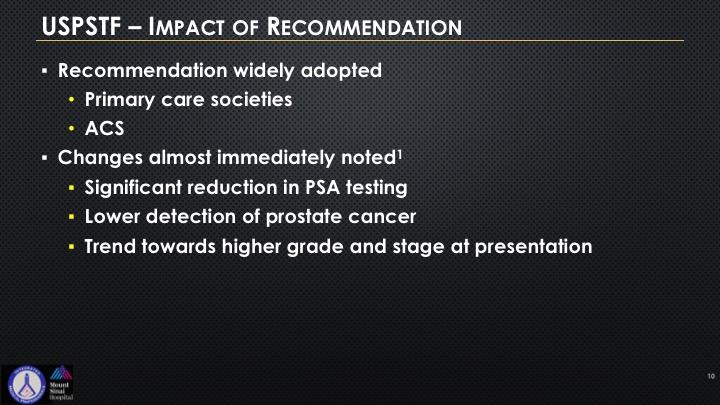
So recommendation was widely adopted by primary care societies and the American Academy of Family Physicians. They have it on their—it gives them coverage medicolegally, “Don’t check PSA.” American Cancer Society widely adopted this as well. Changes almost immediately noted a significant reduction in initial testing, a lower detection of cancer. Articles have been written that there appears to be a trend towards higher grade and stage at presentation.
USPSTF—Regulatory Actions#
In 2015, what you can see here is the NQF, National Quality Forum, and again this is acronym word salad, proposed the ECQM, and this was that non-recommended PSA-based screening is part of a measure applications partnership. And so there was an actual recommendation that was being lobbied on the Hill that there would be a 2% penalty of Medicare reimbursement if you ordered a PSA. We at the LUGPA level put forward an enormous effort and got bipartisan support to have this be left, and then CMS very quietly withdrew this as an ECQM measure.
USPSTF Transparency and Accountability Act#
So where are we today? This is a bill that is up in front of Congress. It’s being sponsored by it’s H.R. 539, sponsored by Marsha Blackburn and Bobby Rush. This is the USPSTF Transparency and Accountability Act, requiring balanced representation of primary and specialty care providers and other stakeholders. Believe it or not, we had to pull back from saying we actually wanted for sure someone from urology, and that became a problem because when it came to all of their other preventative issues, then every specialty would somehow have to be put in there, but that there would be a requirement for public comment on these proposed research plans, require a 60-day comment period of all of these services, require consultation with the relevant stakeholders, and repeals the secretary’s ability to deny Medicare coverage for USPSTF recommendations. All of these things are currently not within the government purview. So we have broad bipartisan support in the House and in the Senate. Senator Cassidy in Louisiana is a big supporter, as is Senator Heitkamp in North Dakota.
USPSTF—Reconsidering?#
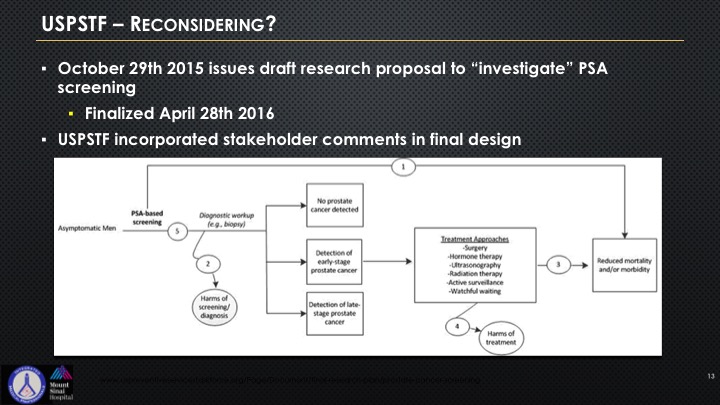
So the USPSTF came back and they said, well, okay—they kind of put this algorithm out there, and quite frankly it’s a bit of an attempt to sort of give I think some respect to all of this lobbying that’s been going on by LUGPA, by AUA, really the two organizations have really come together on this to try to get some better feedback, and you can see this algorithm if you look at it is really rather basic.
USPSTF Reconsidering? Not so much#
So essentially they’re reconsidering but not really that much. We know that they’re on the Hill right now lobbying to basically try to keep intact as much of their power and authority that was not the original intent going back to the early beginnings of this presentation and slide that they are attempting to appear more transparent. They have consulted certain selected specialists who have to sign a non-disclosure confidentiality agreement that they cannot share and we actually know who these folks are, but they are not allowed to share who has spoken with them, or the people who speak with them are not allowed to reveal, and they’ve consulted selected patient organizations. And there’s no disclosure on how they were selected. There’s no disclosure as to the consideration given to the input, and as I said they signed non-disclosure agreements. And they are actively lobbying basically issues and areas on PSA which we’re going to be talking about and the concepts of patients at risk for prostate cancer and who needs treatment, who doesn’t, and there’s not a single urologist on that panel.
USPSTF—Current Status of Prostate Cancer Recommendation #
The current status is to reverse potentially the course and issue a Grade “C” for 55-69 still keep the “D”. Don’t get it for anybody over 70, and they are beginning to acknowledge that there are at-risk populations. The proposal is yet to be finalized, and sadly this is really sort of a tempest and a teapot for all of us. This gets very little media attention, and we’re trying to change that. So at the LUGPA level in addition to our lobbyists, in addition to our legal consultants, we have dedicated energies, and efforts, and resources of marketing to the right sort of media organizations on the Hill.
USPSTF—Summary#
So the task force actions, it’s not just a medical issue. It affects reimbursement, and it’s a real interesting example of government actions producing unintended consequences not only for the healthcare of our patients who are first and foremost our north star but also our ability to get reimbursement. Minor tweaks to processes to avoid regulatory oversight are not really that sufficient. And this really stresses the need for engagement not only in the scientific aspects but the legislative and political realm, and that is a big part of what the mission and vision is of LUGPA and our health policy.
ABOUT THE AUTHOR
Neal D. Shore, MD, FACS, is the Medical Director at Carolina Urologic Research Center in Myrtle Beach, South Carolina. Dr. Shore also serves as a Clinical Urology Specialist at Atlantic Urology Specialists, LLC, also in Myrtle Beach, South Carolina. He is the Director of START GU Oncology Center of Excellence at the START Center for Cancer Research. He is an internationally recognized expert in systemic therapies for patients with advanced urologic cancers of the prostate, kidney, and bladder.