L. Michael Glode, MD, FACP, FASCO, presented “The Role of Targeting DNA Repair with PARP” at the International Prostate Cancer Update on January 27, 2018 in Beaver Creek, Colorado
How to cite: Glode, L. Michael . “The Role of Targeting DNA Repair with PARP” January 27, 2018. Accessed. [date accessed] https://grandroundsinurology.com/The-Role-of-Targeting-DNA-Repair-with-PARP/
Summary:
L. Michael Glode, MD, FACP, FASCO, discusses the mechanism of action of PARP, the basic science behind Synthetic Lethal mutations, clinical trials for PARP inhibition in prostate cancer, and the importance of testing for DNA repair mutations.
The Role of Targeting DNA Repair with PARP – Transcript
Click on slide to expand
Lecture Outline
All right. So the questions are what is PARP anyway? How does it work? Basic science of synthetic lethal mutations, and does it matter in the clinic? And what about prostate cancer?
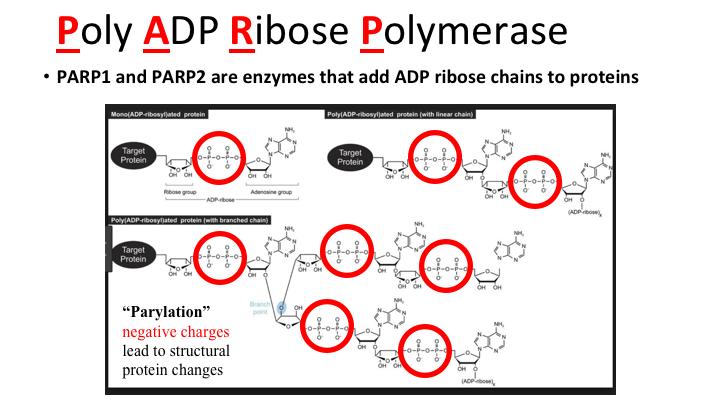
Poly ADP Ribose Polymerase
This is a really interesting story. As most of you know, in the last 20 years or so we’ve had tyrosine kinase inhibitors and they add phosphate groups to tyrosine on proteins that making negative charge, and thus altering function of the protein; dimerization can occur and so forth. Well, it turns out that Poly ADP Ribose Polymerase, PARP, does much the same thing, adding negative charges, phosphate groups, but rather than just a single phosphate, multiple phosphates. As with TK’s there is a target protein, and it can be histones and DNA or for example diphtheria toxin A chain which is a PARP that ADP ribosylates Elongation Factor 2, and that has been an area of interest of mine. The key thing to remember is how potent this can be, so via this one PARP enzyme, DTA, a single molecule of diphtheria toxin can kill a cell. So this can be a very potent effect, but you can start adding more and more chains and eventually get more and more negative charges and that can affect the histone interaction, and recruit other function proteins to DNA repair areas. So this is called parylation, and it’s negative charges leading to structural changes in protein.
Mechanism of action of PARP1 – Synthetic lethality
Now, in the situation of using it in cancer therapy, and we’re going to talk a lot about BRCA1 and 2 in a little bit here, the first thing we want to go through is synthetic lethality, and this means that you have, let’s say this is BRCA1 or 2, and it does not cause cell death, but it does lead to cancer because you get mutations. If you had another mutation in a DNA repair pathway, the cell could still survive, but if you get both of these mutated, or knocked out in the case of BRCA1, and now you add a PARP inhibitor, it leads to cell death. So this is how that looks. You have some patient with any number of DNA repair pathways, and we’ll look at those in a second, and they have mutations in the intrinsic germline or a somatic mutation, their tumor has developed a mutation in DNA repair, and this is PARP and it has zinc fingers that can recognize a single strand break, and attach to the histones associated with this protein and begin doing its enzymatic thing. First thing that happens is it refolds itself, etc., and brings in the catalytic domain to start doing this parylation that we just talked about using NAD as the substrate and adding ADP riboses to this, and that recruits other factors and you start getting repair of the DNA. The PARP1 inhibitors actually trap this so that you can’t go through this pathway and re-establish the uninhibited PARP. So the PARP inhibitors sort of trap this in this configuration and it adds the second mutation that leads to cell death. So that is how PARP inhibitors work.
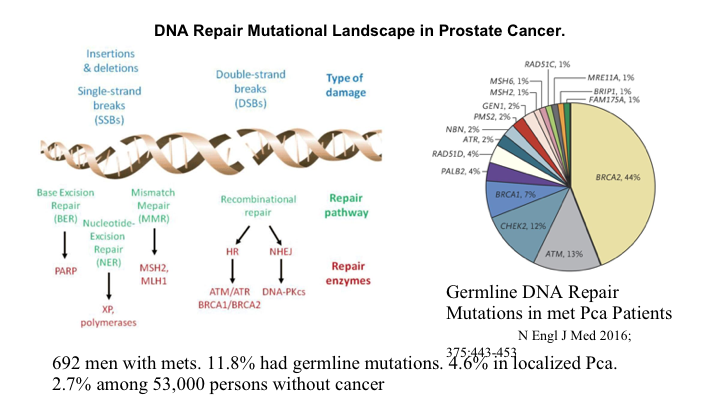
DNA Repair Mutational Landscape in Prostate Cancer
So how does this relate to cancer in general, and prostate cancer in particular? Well, we all know that there are lots of mutations that occur in all cancers, and they can be characterized as repair pathways, as double-strand breaks rather insertions, deletions, different types of damage with different types of repair pathways, and base excision repair is where you take out the base that is mutated and put a new one in. The double strand mutations can occur after a single-strand mutation if you don’t repair things. So, this is a nasty thing that starts happening, and there are a whole series of genes related to DNA repair, and if you have germline mutations in any of these genes, you are at increased risk. I found this to be incredibly interesting. So they’ve looked at in this New England Journal article, published two years ago, 692 men with metastatic prostate cancer, and 12% of those had germline mutations as opposed to only 5% or so with localized prostate cancer. So this means that these people with germline mutations who develop prostate cancer, their cancer is worse and metastasizes more early. You’re walking around with 53,000 people looked at and 2.7% of us have a germline mutation in one of these many DNA repair pathways.
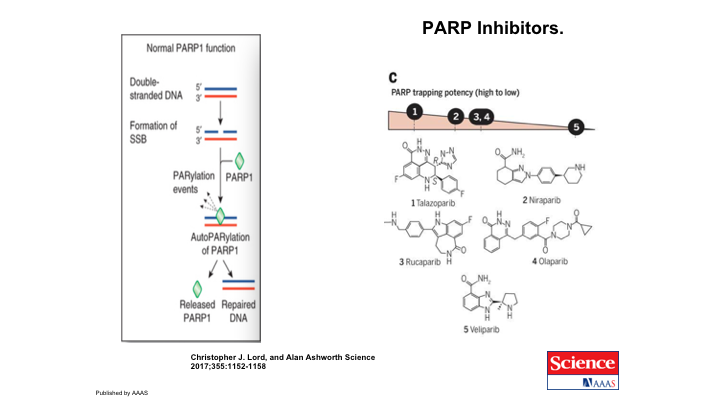
PARP inhibitors
So the PARP inhibitors were developed, as I showed you, to interrupt this pathway. Here is the simple version of what we just looked at, the normal PARP1 function, single-strand break. You have parylation events that lead to repair. The PARP inhibitors prevent that repair. There are a number of inhibitors, small molecules, that have been developed to work this way, and we will look at olaparib as the one that has been evaluated in prostate cancer. Each PARP inhibitor has a different level of PARP-trapping potency. And all of them are being looked at in prostate cancer.
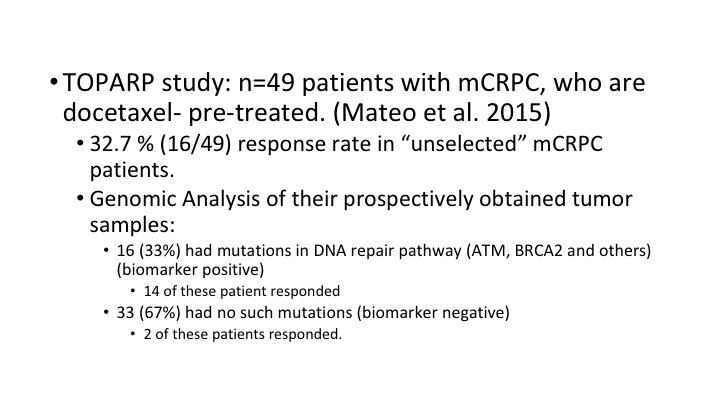
TOPARP study
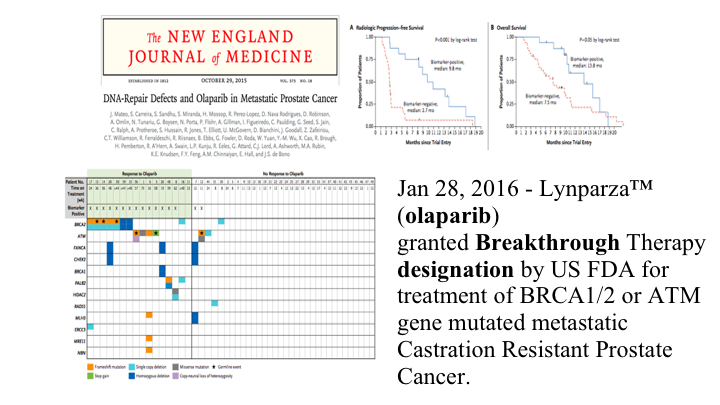
So this TOPARP study had 49 patients with metastatic prostate cancer who had been treated with at least one of the second-generation hormones, hormone blockers that you’ve heard about, and were also docetaxel pre-treated. They then had—they treated everybody in these unselected patients but looked at in retrospect what the tumors had in the way of DNA repair mutations. So in their genomic analysis of their prospectively obtained tumor samples, 16 of the patients had mutations in the DNA repair pathway, and 33 had no such mutations, Then you looked at what was the response rate, and 14 of the 16 patients who had the repair pathway mutations responded, as opposed to only 2 of the 33 patients who didn’t have these mutations. So, this is personalized therapy at its best, and that’s why this has generated a lot of excitement.
The New England Journal
These are the data sort of spread out, and looking up here this is the radiographic progression-free survival in the patients who had the repair pathway mutation versus the patients who did not, and this is their overall survival. So a substantial difference, and because of this study, olaparib was granted breakthrough therapy designation by the FDA in 2016. However, as best I can tell it is still not approved for treatment so it is probably not covered by third parties. This is the different mutations that we looked at in that pie diagram. They were all over the map, and these are the two patients that responded that did not have such repair pathways defects.
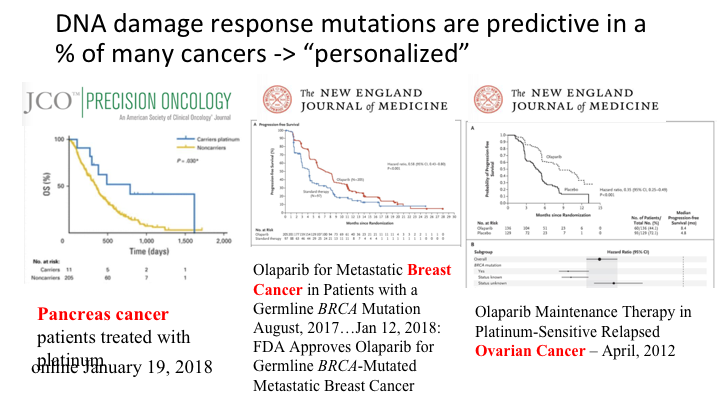
DNA damage response mutations are predictive in a % of many cancers – “personalized”
So this is outside of prostate cancer areas of interest. This is an article that was published just a few days ago in pancreas cancer patients, where the same sort of analysis is recurring. These are the carriers of a DNA repair pathway mutation versus the non-carriers with very significant P values. This is in metastatic breast cancer, where in August of last year germline BRCA mutation patients are now approved for olaparib in treating metastatic breast cancer. And this is the same drug in platinum-sensitive relapsed ovarian cancer, where it has been approved, and a recent study showing that you can prolong the survival of platinum pre-treated patients in this situation.
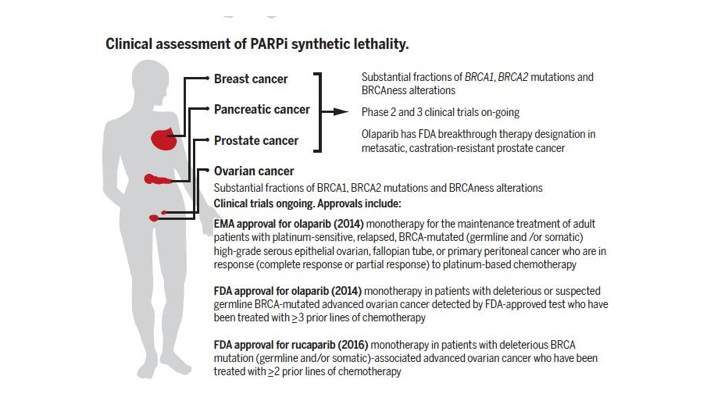
Clinical assessment of PARP1 synthetic lethality
So the clinical assessment of PARP inhibitor synthetic lethality, which we’ve talked about, is now important in breast cancer, pancreatic cancer, prostate cancer, and it’s approved in ovarian and breast cancer, and substantial fractions of these patients have ongoing clinical trials of various sorts. There have been FDA approvals in a few of the other diseases.
PARP Inhibition in Prostate Cancer
PARP inhibition in prostate cancer has breakthrough designation. It will be, if they go with the usual kind of language, indicated for patients who have metastatic disease who have either BRCA1 or 2 or ATM mutations, either in the tumor or germ line, and have received a prior taxane and at least one second generation hormonal therapy. This is under expedited review. The dose is 300 mg twice a day given orally. And the side effects are fairly significant. In one of the studies, not in prostate cancer, 11% of patients stopped taking it because of toxicity. Fatigue and asthenia in as high as 70% of patients. Nausea is frequent. Vomiting less frequent. Low white counts and anemia, and then rarely this can induce myelodysplastic syndrome or acute myelogenous leukemia, pneumonitis and fetal harm is expected but hasn’t been shown. So there are a number of clinical trials targeting the DNA damage response and being developed with all of those different drugs that I showed you.
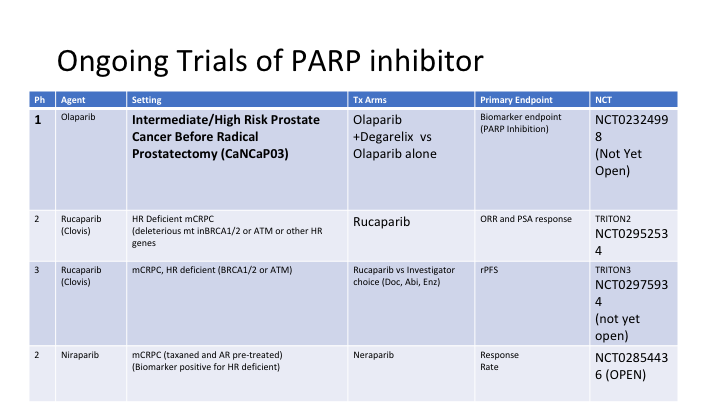
Ongoing Trials of PARP Inhibitor
This is from Dan Petrylak’s slide set. This is olaparib, the one that is the farthest along in development, and these are different trials that are underway with the NCI designations over here. This is rucaparib in hormone refractory DNA repair deficient metastatic prostate cancer, and another trial of that. This is another inhibitor.
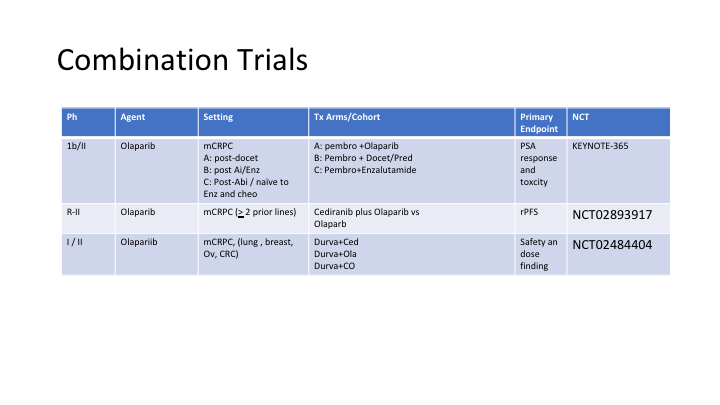
Combination Trials
Here are three more trials with olaparib in different settings combining it with other drugs, because in theory if you have DNA breaks, and then you add this drug to an alkylating agent or even platinum for example, you should be able to accentuate the activity of this drug combination.
Conclusions
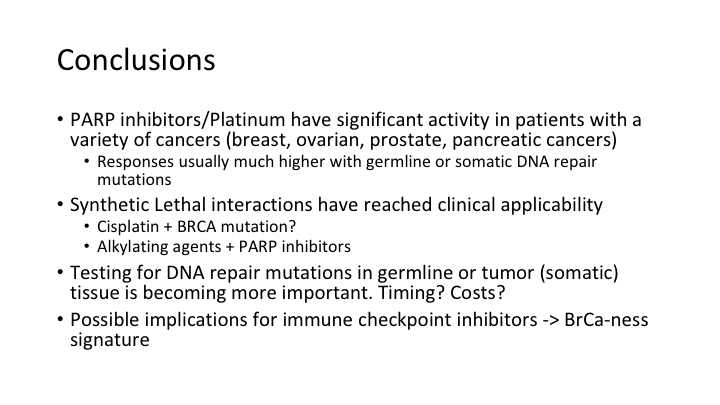
In conclusion, PARP inhibitors or platinums have significant activity in patients with a variety of cancers. These responses are much higher with germline or somatic DNA repair mutations, and this brings into clinical use the synthetic lethal interactions that were hypothesized over 50 years ago, and we now have clinical applicability of this approach to treating cancer. You can combine this with other DNA damaging agents. Testing for DNA repair mutations in germline or tumor tissue is becoming more important. That’s going to be covered tomorrow. When do you do it? How much are the costs of doing that? And then when you do the cost analysis, you have to ask what is the benefit to doing this if it costs a fair amount to do the analysis of DNA repair mutations, but you save giving patients a drug that is going to be ineffective? You may actually save money by doing the repair mutation analysis first. And then there are possible implications for checkpoint inhibitors that I won’t go into, but when you have BRCA or ATM mutations, you get a phenotype of different mutations downstream from that which indicates what is called “BRCAness”, and those patients are the ones that have a much higher burden of mutations, and that in turn leads in some studies to more abnormal proteins being released and increased sensitivity to the checkpoint inhibitors, which as you know in diseases like melanoma have been dramatic, and we are hoping to get there in prostate cancer one day.