Testosterone Replacement and Focal Therapy – Summary
Mohit Khera, MD, MBA, MPH, reviews the literature surrounding the safety of testosterone replacement therapy (TRT) following prostate cancer treatment, including after brachytherapy, EBRT, and radical prostatectomy (RP). He also discusses the concept of bipolar androgen therapy (BAT) and the importance of TRT for erectile preservation post-RP.
When are Physicians Comfortable with Administering Testosterone Replacement Therapy?
Four percent of men under the age of 50 and 8% of men older than 50 suffer from hypogonadism. It is common for these hypogonadal men to have prostate cancer, undergo focal therapy, and then seek TRT. In order to assess if this is safe for these patients, urologists must observe the response to TRT in men who have undergone other prostate cancer treatments, including radical prostatectomy (RP), radiation, and brachytherapy. In the past, few urologists would agree to the idea of testosterone replacement following RP. However, in a survey by Millar et al. from 2016, the majority of participating urologists said it is safe to prescribe TRT following RP, radiation, and brachytherapy.
Testosterone Does Not Increase Risk of Prostate Cancer
Data shows no difference in incidence of prostate cancer, changes in PSA, or urinary symptom scores between men on TRT and men not receiving testosterone, supporting this change in attitude toward TRT. For example, the RHYME study observed hypogonadal men receiving and not receiving TFT. Out of all participants, 55 underwent prostate biopsy for suspected prostate cancer. The proportion of positive biopsies was essentially identical in men with on TRT (37.5%) compares to those not on TRT (37%). This suggests testosterone has no influence on prostate cancer risk. Furthermore, Dr. Khera believes there is not study to date showing that men receiving testosterone have a higher incidence of prostate cancer than those not receiving testosterone.
Testosterone Treatment Following Prostate Cancer Treatment
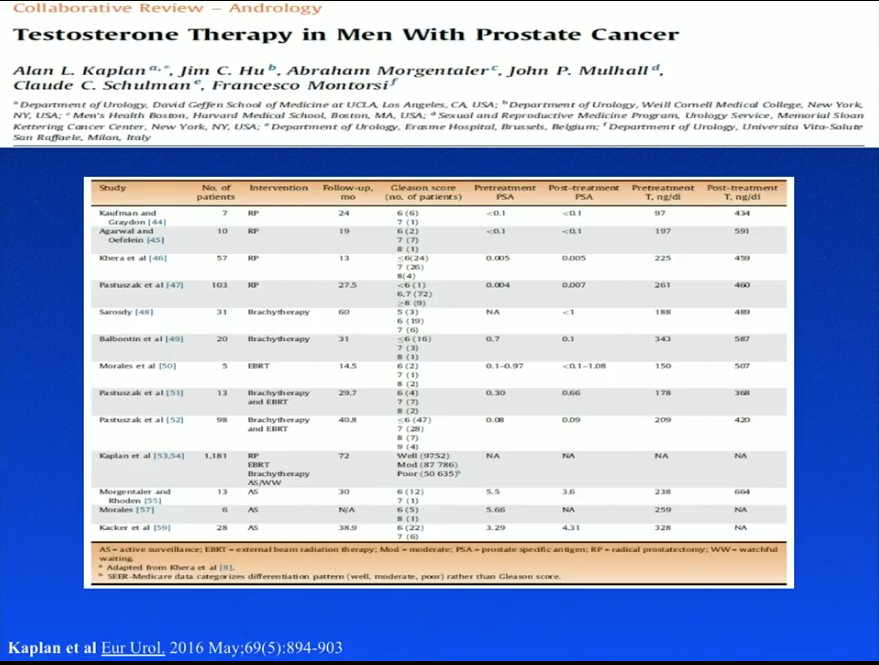
The RHYME study did not expressly observe TRT in prostate cancer patients, and specifically excluded men who had undergone RP. To evaluate patients’ response to TRT after prostate cancer therapy, Dr. Khera summarizes the literature regarding following RP, brachytherapy, external beam radiation therapy (EBRT), and active surveillance (AS). While this literature is favorable to TRT after prostate cancer therapy, it is important to note that they are all small, non-placebo controlled, retrospective studies.
TRT Following Brachytherapy
Sarosdy et al. observed 31 men who received TRT, starting a median of 2 years following prostate brachytherapy, for a median duration of 4.5 years. Serum testosterone levels rose from a median of 188 ng/dL to a median of 498 ng/dL. No patients in this study stopped TRT because of cancer recurrence or progression. Similarly, Balbontin et al. observed TRT in patients after brachytherapy. The majority of the patients had a Gleason score of 6 or higher. At a follow-up of 31 months, the patients showed improved erectile function, but no prostate cancer recurrence.
TRT Following EBRT
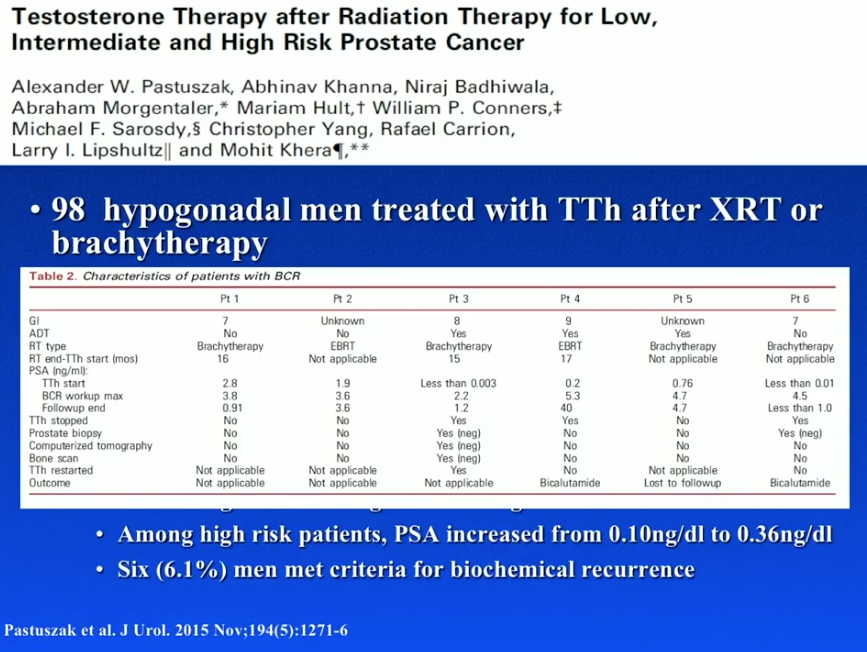
A study by Morales et al. assessed TRT following EBRT in 5 hypogonadal men. TRT increased testosterone levels from 1.1-9.2 nmol/L to 8.5-32.4 nmol/L. At a follow-up of 14.5 months, one patient had a transitory increase in PSA level, but none had levels over 1.5 ng/mL. Pastuszak et al. performed a retrospective review of TRT in 13 hypogonadal men after either EBRT or brachytherapy. At a follow-up of 29.7 months, patients had a significant increase in testosterone levels, but saw no significant increases in PSA or prostate cancer recurrences.
TRT Following RP
Although multiple studies showed no risk associated with TRT after RP, a recent study by Pastuszak et al. tested thus and found 4 PSA recurrences. The study reviewed 103 hypogonadal men treated with TRT following RP and 49 eugonadal, post-RP controls. Of patients in the TRT group, 26 had high-risk prostate cancer with Gleason score of 8 or higher, positive surgical margins, or positive lymph nodes. These high-risk men also requested testosterone supplementation. Results showed 12 biochemical recurrences only in high-risk patients 36 months after RP. Four of those biochemical recurrence were in the TRT group, while 8 were in the control group. Interestingly, this means, out of 26 high-risk patients receiving TRT, the biochemical recurrence rate 36 months after radical therapy was only 15.3%. This is quite low relative to what is expected of a high-risk population 3 years following RP.
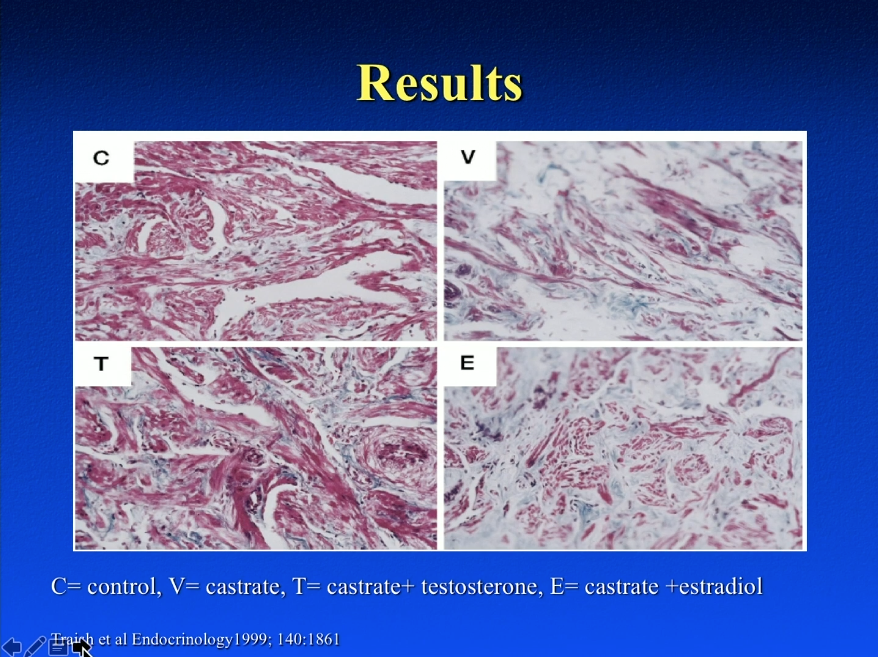
Testosterone May be Protective of Prostate Cancer
The surprising results regarding recurrence rates from the aforementioned study, as well as other basic science data, suggests testosterone may be protective of prostate cancer. For example, Hatzoglou et al. supports that membrane androgen receptor activation induces apoptotic regression of prostate cancer cells. Basic science data from Sonnenschein et al. shows that androgens are able to trigger inhibition of prostate cancer cell proliferation. Most notably, a study by Chuu et al. showed that androgen causes growth suppression and then reversion of androgen independent tumors to androgen dependent tumors on a mouse model.
Bipolar Androgen Therapy (BAT)
Sam Denmeade, MD, a physician from the Johns Hopkins Kimmel Cancer Center, approaches treating metastatic prostate with an unconventional method of prescribing high doses of testosterone. Dr. Denmeade published a study in 2015 observing 14 patients with castration-resistant prostate cancer (CRPC) maintained on androgen deprivation therapy (ADT). The patents also received a combination of testosterone cypionate (TC) and etoposide every month for 3 months. Results showed PSA reduction in 50% of patients and significant improvement in metastatic disease as seen on radiograph. Dr. Denmeade named this concept of treating prostate cancer with ADT plus high doses of testosterone bipolar androgen therapy (BAT). Subsequently, he conducted the Bipolar Androgen Therapy for Men With Androgen Ablation Naïve Prostate Cancer (BATMAN) study, observing 29 asymptomatic hormone sensitive prostate cancer patients. These patients either had a low metastatic burden or non-metastatic disease with biochemical recurrence. They received TC every 4 weeks for 3 months following 6 months of ADT. 59% of these men met the primary endpoint of having PSA levels less than 4 ng/dL after 18 months. Additionally, results showed a significant improvement in quality of life (QOL) and erectile function.
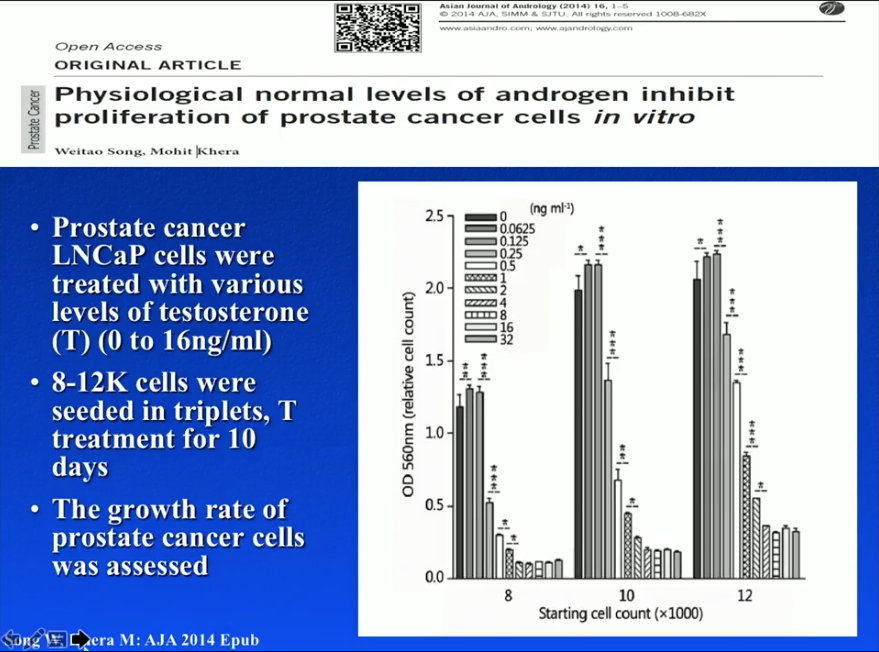
LNCaP Cells
Dr. Khera and a group from Baylor College of Medicine conducted an in-vitro experiment in 2014. Dr. Khera and his team seeded 8,000, 10,000, or 12,000 LNCaP cells into different petri dishes. Physicians treated each petri dish with varying levels of testosterone (0 to 16 ng/mL) for 10 days. Results showed that cells treated with no testosterone had low proliferation levels, while concentrations of testosterone lower than the optimal androgen level promoted proliferation. However, higher, optimal concentrations of testosterone led to greater levels of cell growth suppression. In other words, castration and eugonadal levels of testosterone of may inhibit prostate cancer cell proliferation, while hypogonadal levels may be dangerous.
First Randomized, Placebo Controlled Trial for TRT Following Radical Prostatectomy (RP)
As all the currently available data for TRT for penile rehabilitation comes from small, retrospective series, Dr. Khera and a group from Baylor College of Medicine began a randomized, placebo controlled trial observing TRT in hypogonadal men following radical prostatectomy.At first, the FDA has given approval to begin this trial on the condition that the patients received TRT a year after RP. Due to the importance of beginning a testosterone rehabilitation regimen early after RP, Dr. Khera compromised with the FDA to start participants of the trial on TRT 3 months following RP. Eligible patients had to have undetectable PSA values on two consecutive occasions separated by 4 weeks within those 3 months. Also, patients may not have a Gleason score higher than 3+4 according to the exclusion criteria.Hopefully, results from this randomized, placebo controlled trial will provide a definitive answer regarding the risk of prostate cancer progression and recurrence with TRT following RP.
Risk of Occult Prostate Cancer
A retrospective study by Morgentaler et al. observed 13 symptomatic testosterone deficient prostate cancer patients who elected AS. Of these men, 12 had a Gleason score of 6 and one had 3+4. These men received TRT for a mean duration of 23.5 months following initial prostate cancer diagnosis. Results showed no significant change in PSA or prostate volume, no cases of progression, and no cancer identified on 54% of patients in follow-up biopsies. Dr. Khera emphasizes the fact that there has been no published studies showing men treated with TRT have a higher incidence of prostate cancer than those not receiving TRT.
Efficacy of TRT for Erectile Preservation Following RP
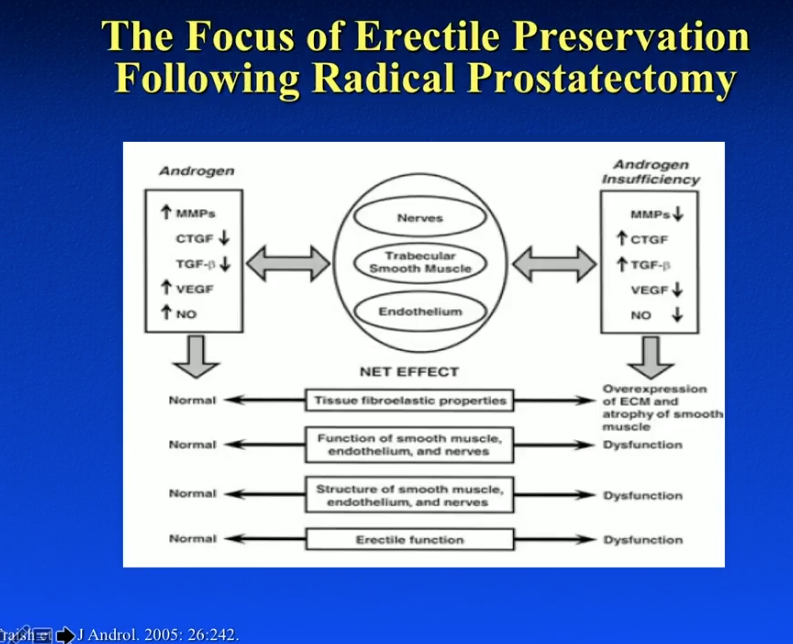
Hypogonadal prostate cancer patients have a significant disadvantage in recovering erectile function following RP compared to eugonadal patients. Testosterone is critical in overall recovery of erectile function. It regulates nitric oxide synthase and phosphodiesterase type 5 activity within the penile tissue. Additionally, it is important for penile nerve function and veno-occlusive erectile function. In fact, a hypogonadal state will result in fat deposition within the tunica albuginea, as well as decreased apoptosis of cavernosum smooth muscle. Hypogonadism can be a true cause of erectile dysfunction in many patients.
Conclusions
Dr. Khera concludes that currently, no convincing evidence suggests that TRT promotes the initiation of prostate cancer in hypogonadal men. TRT may promote low prostate cancer recurrence rates in hypogonadal men. Therefore, physicians can consider TRT in men with a history of focal therapy. A large, randomized, placebo-controlled trial is necessary for assessing the effects of TRT on prostate cancer. Finally, androgens may play a key role in recovering erectile function following RP.
ABOUT THE AUTHOR
Mohit Khera, MD, MBA, MPH, is a board-certified urologist specializing in male infertility, male and female sexual dysfunction, and declining testosterone levels in aging men. Dr. Khera’s research focuses on the efficacy of botulinum toxin type A in treating Peyronie’s disease as well as genetic and epigenetic studies on post-finasteride syndrome patients and testosterone-replacement therapy.
Dr. Khera earned his MBA and MPH from Boston University before earning his MD from the University of Texas Medical School at San Antonio. Dr. Khera completed his urology residency training in the Scott Department of Urology at Baylor College of Medicine (BCM) where he also completed a one-year general surgery internship. After completing his urology residency, he went on to complete a one-year fellowship in Male Reproductive Medicine and Surgery at BCM. Currently Dr. Khera is a professor in the Scott Department of Urology at Baylor College of Medicine, and he holds the F. Brantley Scott Chair in urology. Dr. Khera also serves as the director of the Laboratory for Andrology Research, the medical director of the Baylor Executive Health Program and the medical director of the Scott Department of Urology. He also serves as president of the Sexual Medicine Society of North America.
Dr. Khera has initiated numerous FDA-approved clinical trials. His scientific and clinical experiences have allowed him to thus far give over 400 lectures at scientific meetings throughout the world, publish over 160 articles in peer-reviewed journals, complete 15 book chapters, and edit and write two books in the field of sexual medicine and men’s health. Dr. Khera shares his time and knowledge with the general public. He has been voted several times as one of Houston’s Best Doctors by Health and Sport Fitness Magazine and by Houstonia Magazine and is a frequent guest on such TV programs as Fox News’ “Ask theDoctor.” He also writes a blog on men’s health for the Houston Chronicle newspaper.