Phillip J. Koo, MD, presented “Fluciclovine 18F PET-CT Impact on Clinical Management of Recurrent Prostate Cancer” during the 27th Annual Perspectives in Urology: Point Counterpoint on November 9, 2018 in Scottsdale, Arizona.
How to cite: Koo, Phillip J. “Fluciclovine 18F PET-CT Impact on Clinical Management of Recurrent Prostate Cancer” November 9, 2018. Accessed Apr 2025. https://grandroundsinurology.com/fluciclovine-18f0-pet-ct-impact-on-clinical-management-of-recurrent-prostate-cancer/
Fluciclovine 18F PET-CT Impact on Clinical Management of Recurrent Prostate Cancer Summary:
Phillip J. Koo, MD, discusses conventional prostate cancer imaging techniques and the need for advanced imaging techniques in order to better detect recurrent disease. He then argues that fluciclovine (18F) positron emission tomography/computed tomography (PET/CT) could satisfy this unmet need.
Abstract:
Prostate cancer typically recurs in 40%-50% of patients after initial treatment. In order to effectively manage recurrent prostate cancer, it is essential to have accurate, reproducible, validated, accessible, and cost-effective imaging modalities. Imaging helps guide prostate cancer management, since the presence, volume, and distribution of disease have implications for therapeutic choices. Evidence shows that conventional imaging techniques that emerged in the 1970s, including bone scans, CT, and MRI, have limited sensitivity and specificity for detecting recurrence. Therefore, a demand in the urological community for optimized imaging modalities has emerged over the last decade. Fortunately, next generation PET/CT imaging may allow for detection of metastases that conventional imaging cannot detect.
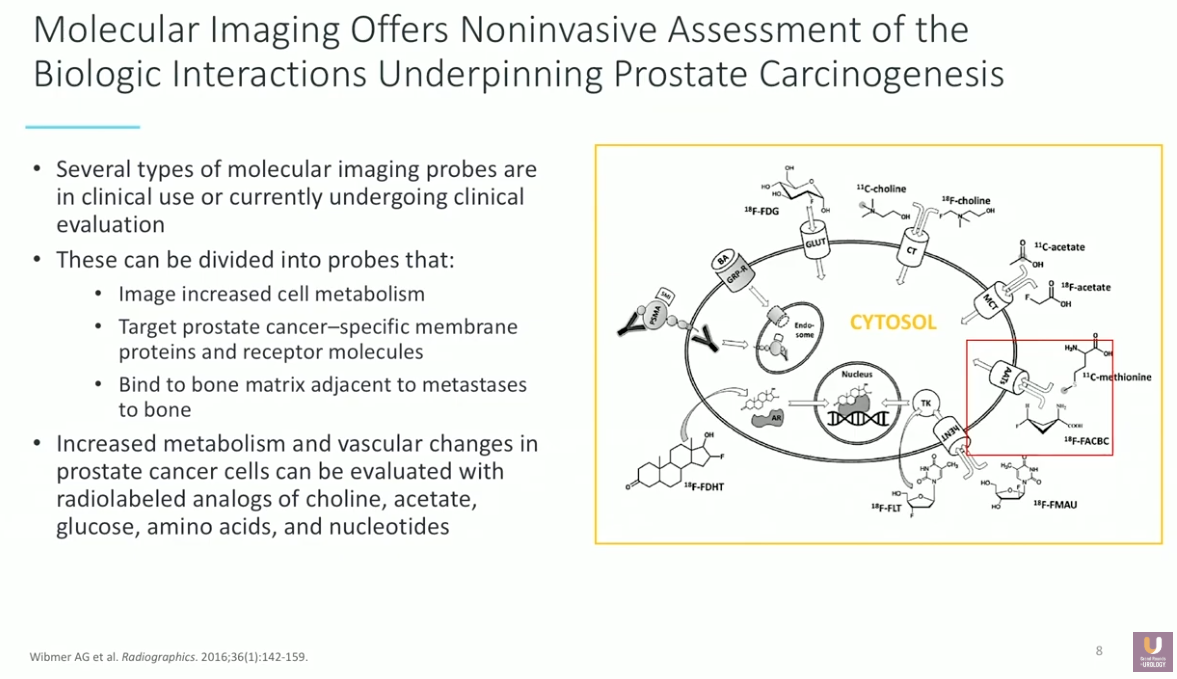
The rationale behind PET/CT imaging is that many positron-emitting isotopes can target certain prostate cancer-specific membranes, proteins, and receptors. This presentation focuses on the molecular imaging agent fluciclovine (18F), an amino acid analog that detects increased cellular metabolism. The FDA approved this agent in 2016 for PET/CT imaging in men with suspected prostate cancer recurrence.
This has significant ramifications for the management of recurrent prostate cancer, as the 18F isotope has a half life of about two hours, allowing for widespread accessibility.
The discussion will review the two registrational studies for fluciclovine (18F) that led to its FDA approval, the LOCATE trial, as well as the RADAR III expert consensus. It will also comment on commercial coverage issues for this agent.
Following his presentation, Dr. Koo responds to questions from Daniel P. Petrylak, MD, Raoul S. Concepcion, MD, and Steven E. Finkelstein, MD.
About Perspectives in Urology: Point Counterpoint
Perspectives in Urology: Point Counterpoint (PCP) is an annual CME-accredited conference devoted to discussing and debating the latest topics in men’s health, general urology, and genitourinary cancers. The conference’s format includes more than didactic lectures. It also includes debates, point-counterpoint discussion panels, and unique case-based presentations. Dr. Koo presented this lecture during the 27th PCP in 2018. Please visit this page in order to register for future PCP meetings.
ABOUT THE AUTHOR
Phillip J. Koo, MD, is the Chief of Diagnostic Imaging at the Banner MD Anderson Cancer Center in Phoenix, Arizona. Prior to this, he was Chief of Nuclear Medicine and Associate Professor of Radiology at the University of Colorado School of Medicine in Aurora, Colorado. He completed his fellowship at the Harvard Medical School Joint Program in Nuclear Medicine in Boston, Massachusetts. Dr. Koo is a diplomate of both the American Board of Radiology (ABR) and American Board of Nuclear Medicine(ABNM).