Leonard G. Gomella, MD, FACS, presented “Basic Concepts in Bladder Cancer Immunotherapy ” at the 2nd Annual International Bladder Cancer Update on January 27, 2018 in Beaver Creek, Colorado
How to cite: Gomella, Leonard G. “Basic Concepts in Bladder Cancer Immunotherapy ” January 27, 2018. Accessed Jan 2026. https://grandroundsinurology.com/Basic-Concepts-in-Bladder-Cancer-Immunotherapy/
Summary:
Leonard G. Gomella, MD, FACS, reviews the basic scientific concepts of immunotherapy for bladder cancer, from why BCG is the “gold standard” of treatment and describing the mechanics of augmenting the immune system to eliminate tumor cells, to categorizing therapies into vaccine based, cytokines, monoclonal antibodies, checkpoint inhibitors, and CAR-T cells. He also explains why, generally, immunotherapy is particularly effective in the bladder cancer setting, as opposed to other cancers.
Reveal the Answer to Audience Response Question #1
Finish the sentence- Bladder cancer is a good target for immunotherapy based on:
- A. Ability to instill drugs directly into the bladder.
- B. A high rate of somatic mutations.
- C. Low numbers of T- Cells in bladder tumors gives better immune response.
- D. Systemic immunization with BCG can clear superficial bladder cancer.
Reveal the Answer to Audience Response Question #2
Which of the following statement is true concerning checkpoint inhibition and bladder cancer?
- A. BCG has been shown to work through the PD-1/PD-L1 checkpoint inhibition.
- B. Checkpoint inhibitors are approved for both systemic and intravesical use.
- C. All newly approved checkpoint inhibitors block both PD-1 and PD-L1.
- D. FDA has approved complementary diagnostic, the PD-L1 (SP142) assay with atezolizumab.
Reveal the Answer to Audience Response Question #3
Finish the sentence- In a patient who fails Intravesical BCG therapy with HG NMIBC:
- A. Intravesical interferon alpha is an FDA approved subsequent immunotherapy.
- B. Recurrent bladder tumor should be stained to determine if the cells are CD8 positive or negative to decide if more BCG should be given.
- C. Patient should undergo cystectomy or be offered a clinical trial.
- D. Systemic PD-1 or PD-L1 checkpoint inhibitor should be used.
Basic Concepts in Bladder Cancer Immunotherapy – Transcript
Click on slide to expand
Intravesical BCG Immunotherapy
Immunotherapy is the hot topic, as everyone knows. We’re in sort of a golden area for almost every malignancy concerning immunotherapy. There’s so much that we’ve learned over the last 25 or 30 years, how to take our own innate immunity, and everything that we’re talking about is tweaking our own immune system. The only one that maybe is a little bit different is the CAR-T therapy, but everything else is just an enhancement or an augmentation to our existing immunity.
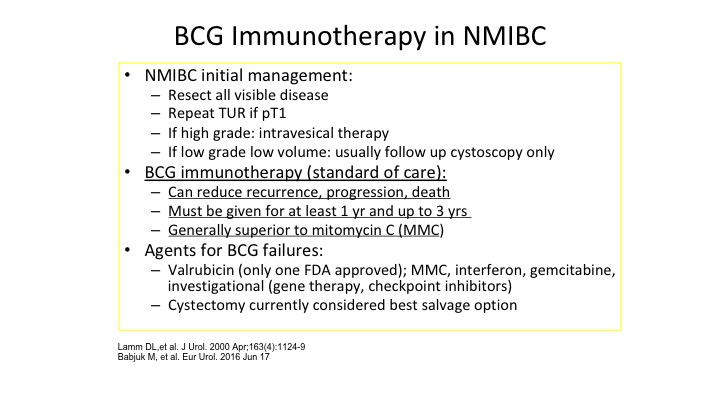
BCG Immunotherapy in NMBIC
So of course BCG is the big dog when it comes to immunotherapy of almost every cancer. It’s the model. It was one of the original ones that out there, where you’re stimulating your own immune system to reject the cancer. The interesting thing is BCG is also being used for melanoma, directly injecting BCG into melanoma has caused many remissions. But BCG immunotherapy everybody knows is our standard of care. It can reduce recurrent/progression. It has to be given at least one year, and probably up to three months for high-risk non-muscle invasive bladder cancer and overall is superior to intravesical chemotherapy. So we set the stage for talking about basic concepts in immunotherapy knowing that we as urologists who would be using BCG extensively since the late 1980s and early 1990s. There surely should be the thought leaders in a lot of this area of immunotherapy.
BCG Intravesical Immunotherapy
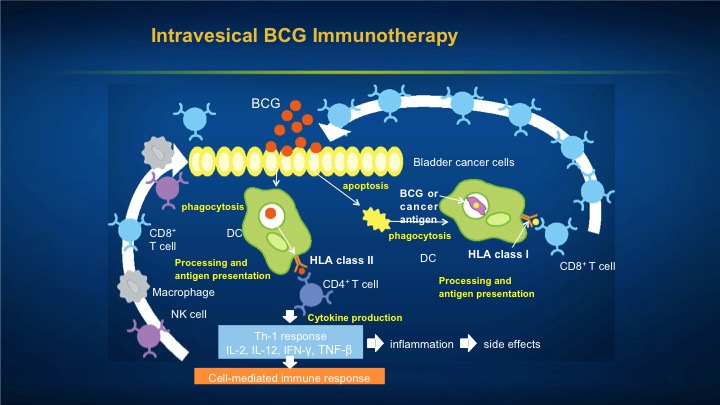
So the mechanism, although we’ve been using it for a long time is really still unclear, but it is clearly recognized as an augmentation to our own immune system and we did actually some work in the early 1990s actually we were the first ones to show that BCG, one of the reasons that it does stimulate the immune system is actually taken up since the gag layer is absent on urothelial cancer, BCG is actually engulfed by the bladder tumor itself, and that is one of the ways that the immune system is stimulated. Again, safety it’s a pretty safe approach, and we recognize if it’s used properly it is a good immunotherapy.
Overview of Cancer Immunology and Immunotherapy
So what I want to do with that background of recognizing the fact that as urologists we are the leaders in immunotherapy. Let’s roll things back now and talk about kind of the basics of immunology and exactly why things like BCG work.
Hallmarks of Cancer
So Dr. Weinberg has really been famous for putting these—the hallmarks of cancer, what makes cancer develop, what makes cancer progress, but one of the big things that Dr. Weinberg has really shown us is the fact that the immune system has a major role not only in clearing cancer but allowing cancer to progress and develop.
Increased Incidence of Cancer in Immunocompromised Patients
So immunocompromised patients, anyone who questions the role of our own innate immune system really has to look at some very significant data that’s out there. We don’t have to convince ourselves but it’s nice to have the data to back up our concept, so if you look at historically those people who were on compromised immune systems, such as renal transplant, liver transplant and others you can see that the significant increased risk of cancer in these individuals suggesting that immunosuppression of your own innate immunity causes or encourage, allows the development of malignancy.
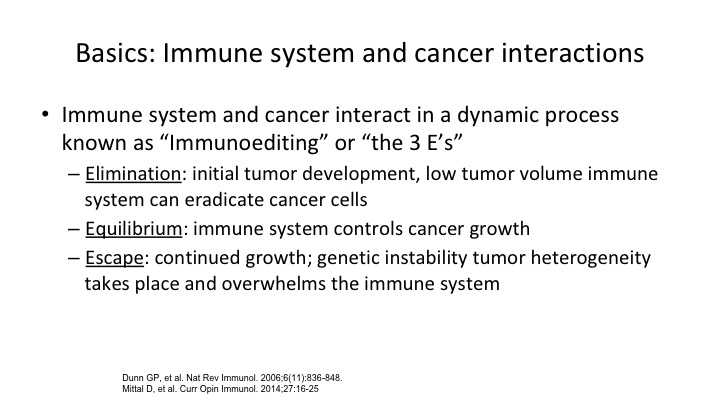
Basics: Immune System and Cancer Interactions
So the immune system has now been broken into sort of three e-components as they’re called, elimination, equilibrium, and escape, and basically how our body interacts with the tumor falls into one of these three categories.
Immune Evasion of Cancer
This is the concept of how our bodies normally will get rid of a tumor through elimination, and in fact it is projected that as we get older, as our immune surveillance declines and our ability to recognize foreign invaders, be it pneumonia, be it herpes zoster, whatever it might be, the reason that older folks get more cancer is because there’s decreased immune surveillance. So what happens is that a tumor can be cleared and eliminated by our intrinsic immune system. Most of us are probably harboring immune cells most of the time, and they just sort of sit in an equilibrium. They don’t get an upper foot. They don’t grow. If you look at our good old friend, renal cell carcinoma, the old famous 1 to 2% spontaneous remission. It probably had to do with the elimination of the tumor by our intrinsic immune system. But escape is what we’re talking about, and escape is when we have things like the checkpoints that get breached by the tumor and allow the tumor to grow. So this concept of escape, equilibrium and elimination has been shown to be operational in most tumors.
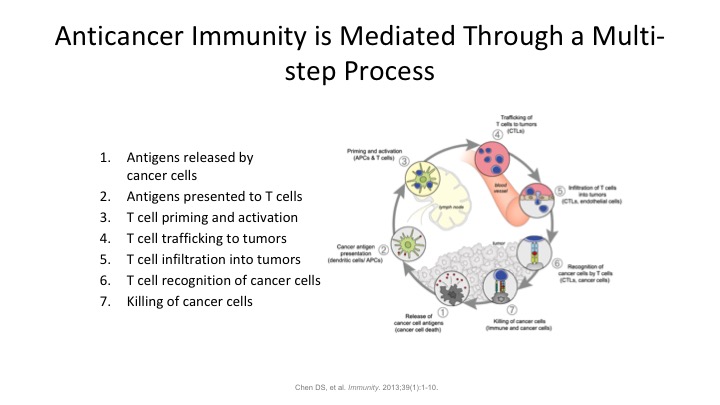
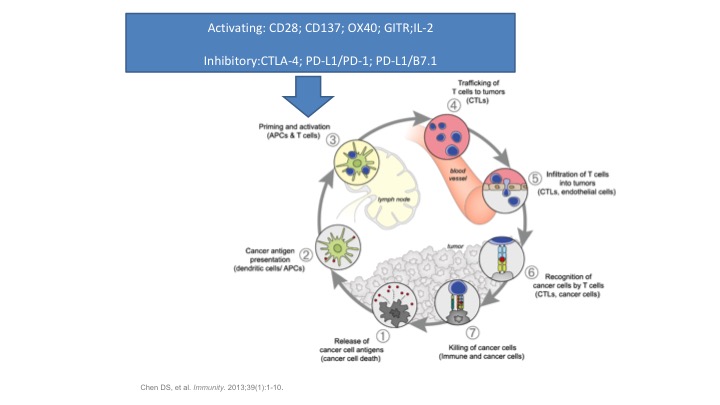
Anticancer Immunity is Mediated through a Multi-step Process
So our anticancer immunotherapy is mediated through multiple steps. There’s not one sort of—it’s not a single bullet theory here. There’s many different phases. So antigens being released by the cancer cells, antigens get presented to T cells. The T cells get primed. They traffic to the tumor. They infiltrate into the tumor. They recognize the tumor, and then they bring in other cells to go ahead, CD8 and CD4 positive cells to go ahead and clear the tumor. So tumor immunology is almost completely dependent on our intrinsic immune system is totally dependent on the activation of our T cells. Bare antigens floating around in the blood stream tend not to be recognized as foreign so the tumors which might be shedding DNA out into the systemic circulation or other cell surface markers are not going to be recognized unless they get trapped by a T cell and then presented to a macrophage, and tell the macrophage, look for this guy, it’s a bad actor, and go ahead and kill it.
Activating: CD28, CD137, OX40, CITRL, IL-2
So we have a lot of players on the field when it comes to our immune system. You have things that activate the immune system, but you also have to have things that slow the immune system down because if the immune system goes wild, you develop autoimmune diseases and you can actually create a lot of problems. And that’s one of the things we’re recognizing now with a lot of the things such as the checkpoint inhibitors. There’s coming in a whole new class of side effects that we’re only now beginning to recognize and figure out how to overcome, but activating and inhibitory factors in this immune response, and I want to focus on the inhibitory ones at the bottom. The CTLA4, the PD1, the PDL1, basically these are the main ones that we are now manipulating in the immune system to treat diseases such as bladder cancer. We’re taking the breaks off the immune system by slowing these inhibitory factors down and allowing our own immune system to go ahead and clear the tumor.
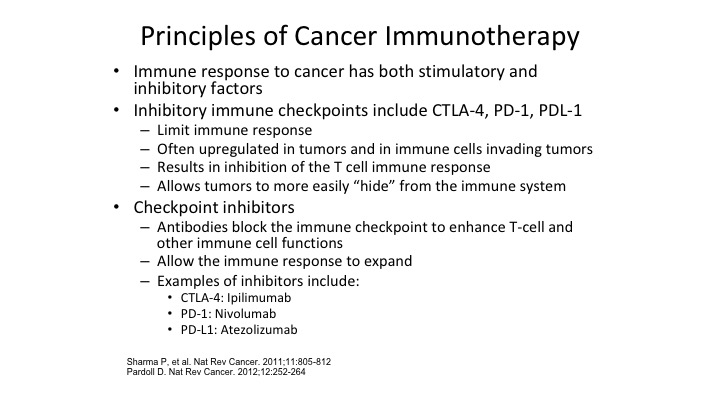
Principles of Cancer Immunotherapy
So basic principles of cancer immunotherapy, the immune response to cancer, as we mentioned has stimulatory inhibitory, what we want to do to treat these cancers, get rid of mostly the immune inhibitory factors. Stimulatory factors, things like IL-2 that we’re all familiar with, and other interferons we’ve used for years, but the big magic ticket that has really happened is understanding how do we take the inhibitory factors and negate them, slow them down to allow our own immune system to take over. Sot he big checkpoint inhibitors, the CTL4, the PD1, the PDL1, the ipilimumab is that CTLA4, which has a big play right now in melanoma, and in combination with tumors such as bladder cancer combining checkpoint inhibitors is going to be the next wave as was addressed earlier, as has been some of the earlier talks. So the checkpoint inhibitors block the immune checkpoint to enhance the T-cells ability to identify and eliminate tumors.
Principles of Cancer Immunotherapy Continued
So when you look at immunotherapy we have two general classes, passive immunotherapy and active immunotherapy. For urologic tumors, passive immunotherapy doesn’t do us a lot of good because for passive immunotherapy you have to continually administer these medications, and as the tumors grow you can’t really keep up with the growth of the tumor and really try to clear it. But there are monoclonal antibodies out there for other diseases which are effective. However, for things like bladder cancer and kidney cancer they tend not to be as effective. However active immunotherapy, where we kick our own T cells into high performance to identify tumors is really some of the most effective approaches that we have. So remember that antigens on the tumor cell need to be presented to T cells, and antigen-presenting cells, and I might have said that backwards a minute ago. The macrophages eat up these tumors. They then process the antigens, stick them outside and tell the T cells please seek these tumors out and destroy them. So that’s this antigen presentation concept. Dendritic cells, of course Dendreon that name sounds kind of familiar, is a way of taking antigen-presenting cells, putting them back in the body and it’s those APCs in Dendreon’s Provenge for example, you put it back into the patient. Those dendritic cells identify prostate cells. By identifying those cells, they then tell the T cells go after this particular tumor.
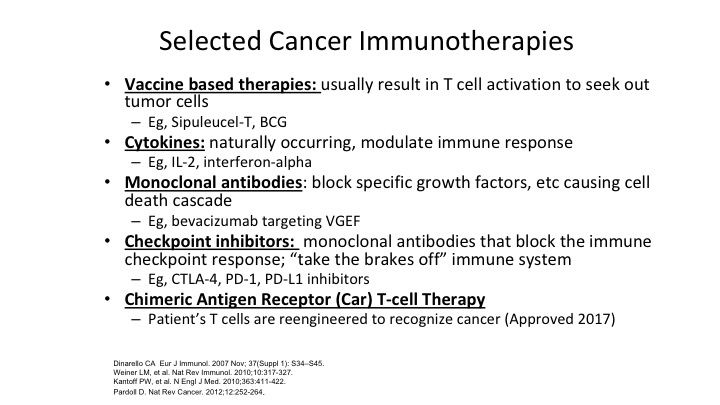
Selected Cancer Immunotherapies
So when you look at specific immunotherapies, they come into these categories, vaccine based, cytokines, monoclonal antibodies, checkpoint inhibitors, and CAR-T cells, or chimeric antigen receptors. The vaccine-based therapy basically we kind of put sipuleucel T and BCG into that bucket because we use those—we use the term vaccine but essentially what you are doing is you are identifying the tumor by injecting more of the tumor factor in the patient so that results in the T-cells finding those tumors out and being killed.
Cytokines are things like IL-2 and interferon. The problem is they are kind of transient in their effect. Yes, they can clear some tumors, but we know that IL-2 if it clears your tumor and that is the only thing that has been found at this point to ever eradicate renal cell carcinoma. It can be effective, but these things tend to need to be chronically administered. Monoclonal antibodies, there are monoclonal antibodies that block growth factors, and again there are certain tumors that are exquisitely responsive to things like bevacizumab targeting VEGF. But when it comes to urothelial and other malignancies, we haven’t been very effective. We have some monoclonal antibodies directed against growth factors for renal cell carcinoma but they have not been that effective in bladder cancer.
Checkpoint inhibitors are technically also monoclonal antibodies but they are different in that they are blocking the immune response, and basically taking the brakes off of the immune system when the tumor is trying to suppress your immune system, these monoclonal antibodies go in and block either the PD1 or the PDL1 receptor and take the breaks off that interaction because these tumor cells are trying to suppress the immune response, and this has been the big breakthrough over the last 5 to 7 years, identifying the PD1 and the PDL1 factors on both the tumor and the T-cells. So these are monoclonal antibodies that block the PD-1 or the PDL1 that we’ll talk a little bit more about in a minute.
Lastly, the hot thing in the world right now is is the CAR-T cell where you are genetically engineering the patient’s T cells to re-introduce genetic material into a patient’s own T cell to go after primarily at this point it’s being looked at mostly in hematologic malignancies, but there’s a lot of work being done in solid tumors right now, and we’ll have to see what direction that goes in. So this is kind of a little bit of a review before we get into the real nuts of the—nuts and bolts of the PD1 and the PDL1.
Intravesical BCG Immunotherapy
So you can see that the BCG can either be taken up by the tumor cell and then recognized as a foreign invader, or it can cause direct inflammation in the bladder because remember GAG, the glucose amino glycans, which is normally protective of the bladder is not on tumor cells. So what happens is these tumor cells are sitting out in the bladder with no protective cloak upon them which is the GAG layer, so what many happens is they actually take up the BCG and then get identified as foreign invaders. The BCG itself sets up cell mediated immunotherapy which also results in some of the side effects.
Nature
But the big thing right here when we talk about immunotherapy is the PD1 and the PDL1 immune checkpoint inhibitors.
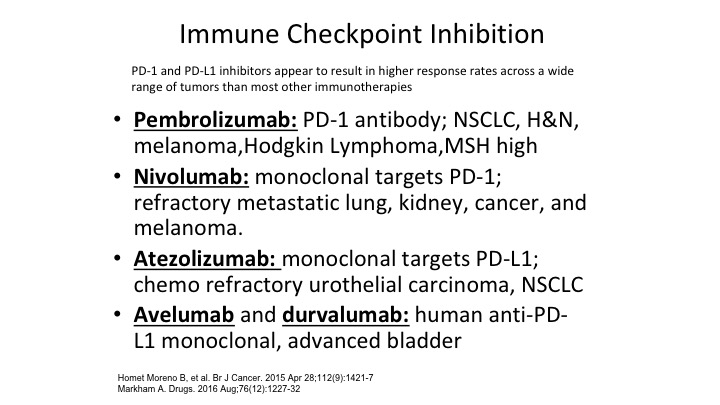
Immune Checkpoint Inhibition
And right now these are the ones that we have, and we have people in the audience like Dan Petrylak who are real experts in this area and done some of the pioneering work, but we now have five different immune checkpoint inhibitors that are available to us in bladder cancer. All of them are either in primary and none of them are for localized disease although as we’ll talk a little bit about, there are a bunch of trials out there looking at these immune checkpoint inhibitors in localized disease. .
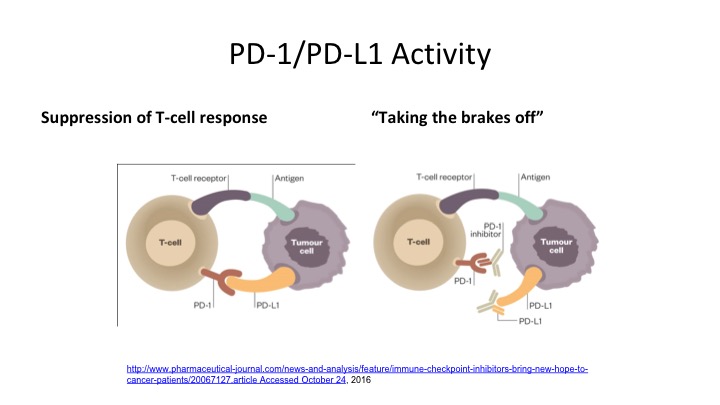
PD-1/PD-L1 Activity
So here is really probably the take-home message of the whole talk, looking at anti-PD-1 and anti-PD-L1 activity. So you can see what happens on the T cell. The PD-1 and the PD-L1 interact. So PD-1 is on the T-cell, PD-L1 is on the tumor cell, and what happens is when that T-cell receptor identifies the tumor cell, it tries to suppress that response, and if you can block that—you either block the tumor ligand or you block the T-cell receptor in order to take the breaks off the immune system because this tumor cell is trying to suppress this anti-tumor effect, and by blocking it either on the ligand side or on the receptor side, it allows that to take the brake off the immune system and allows that T-cell to adequately identify that tumor cell and cause it to undergo apoptosis.
Why is bladder cancer a good target for immunotherapy?
So why is bladder cancer a good target for immunotherapy? You guys did a very good job with this. Bladder cancer has almost as much somatic tumor mutations as things like melanoma, and lung cancer, which tends to be the high, high, the ones if you look at the responsiveness for these immunotherapies, they tend to be ones with a lot of somatic mutations, meaning that they look so wild on their wild on their cell surface markers, it’s a heck of a lot easier for the immune system to recognize them. These high rates of somatic mutations we believe may make the immune response and the immune treatment work much better, and the urothelial cancers express these substances that suppress the immune system such as the PD-L1 ligand. So CD4 and CD8 T cells express inhibitory PD1 to prevent over stimulation of the immune system. So your body is trying to modulate this. Your tumor is trying to suppress it. Your body is trying to turn it on, and by going in favor of your body by blocking the immunosuppressive activity of PD1 or PD-L1 interaction, that is how we hopefully are doing better with clearing these tumors.
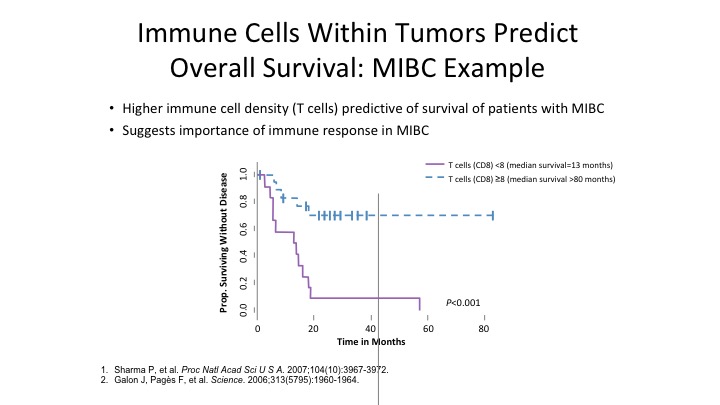
Immune Cells Within Tumors Predict Overall Survival: MIBC Example
So immune cells within the tumor predicts overall response. The more CD8 positive cells you have in muscle invasive bladder cancer, the better the patients do overall from a survival standpoint.
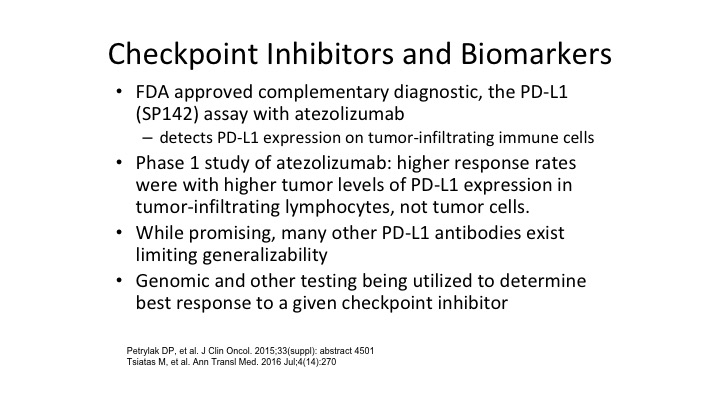
Checkpoint Inhibitors and Biomarkers
Checkpoint inhibitors and biomarkers, Dan will I’m sure comment more about this. It’s not clear that the markers are completely reliable when it comes to identifying PD1 or PD-L1 because many patients in spite of having negative PD-1 or PD-L1 staining still respond to some of our checkpoint inhibitors. There’s a lot of PD-L1 and PD-1 monoclonal antibodies out there so while a lot of this is a research standpoint and companion assays are approved, they are still not as predictive for response as we would like them to what we would like them to be.
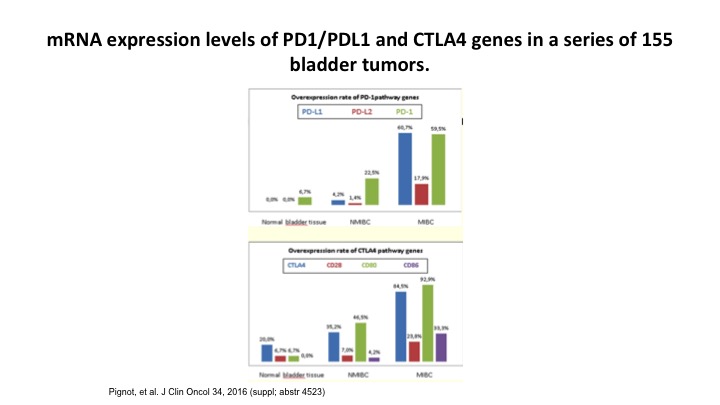
mRNA expression levels of PD1/PDL1 and CTLA4 genes in a series of 155 bladder tumors.
So if you look at bladder tumors, and you look at PD1 and the PDL1 expression, again you can see that they over express. This is from a gene standpoint, not from an immunohistochemical standpoint. This is form a research standpoint, but in fact, and I’ll see what Dan thinks about this. This may actually work its way in and be a more reliable marker for the PD1 and the PDL1 inhibitors.
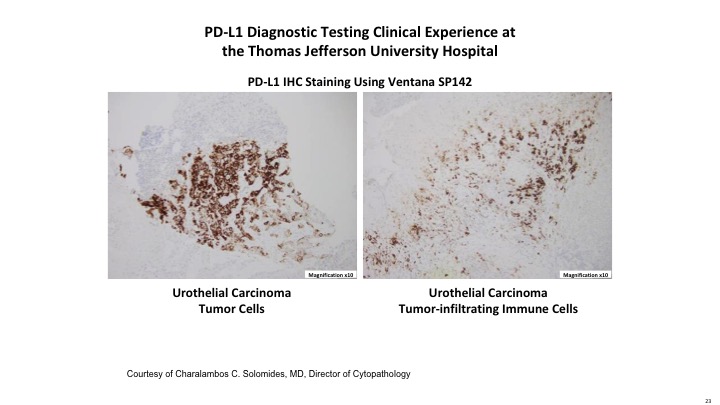
PD-L1 Diagnostic Testing Clinical Experience at the Thomas Jefferson University Hospital
Here is just an example of the Ventana SP142 assay that looks for PDL1 immunohistochemical staining, both on the tumor itself and on the tumor infiltrating cells.
Intravesical Immunotherapies
Lastly, intravesical immunotherapies, we have a whole bunch out there. Most of these right now live in the world of superficial bladder cancer.
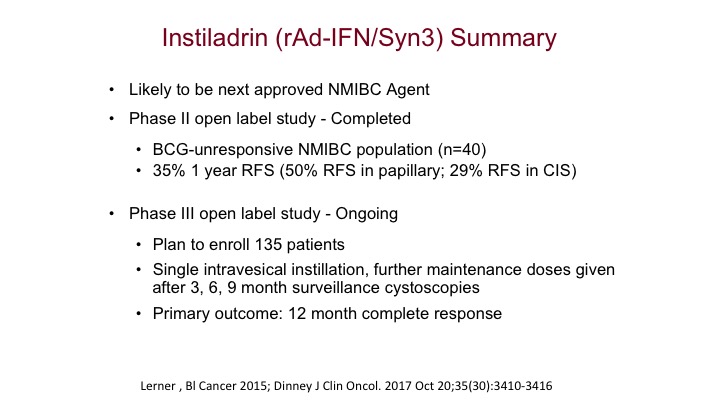
Instiladrin (rAd-IFN/Syn3) Summary
The one and again it’s been spoken about is the Instiladrin, and I think this is the one that’s going to be our closest next immunotherapy specifically approved for intravesical use is probably going to be this recombinant adenovirus interferon vector that was addressed earlier, and we think this will be the next one specifically approved as an immunotherapy in superficial bladder cancer.
Some Current Phase II Checkpoint Trials
Whole bunch of studies out there, dozens upon dozens of studies looking at these things systemically for early disease. So this is going to be the next thing urologists are going to have to be aware of is the systemic therapies going for superficial disease.
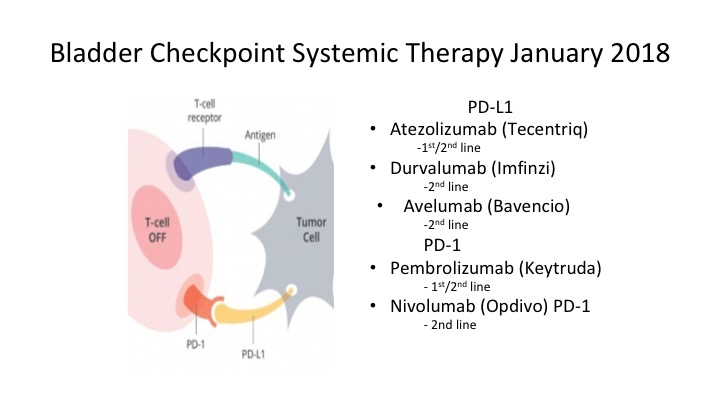
Bladder Checkpoint Systemic Therapy January 2018
Again, where are we today? These are the bladder checkpoint inhibitors. They’re either directed at the PD-L1, either at the ligand or the PD1 receptor and again we sort of have an embarrassment of riches right now. We have a lot of these agents out there and I think you develop familiarity with one of these agents and decide which one you’re most comfortable using.
So I think that is the end of the talk.
ABOUT THE AUTHOR
Leonard G. Gomella, MD, FACS, serves as the Bernard W. Godwin, Jr. Professor of Prostate Cancer, Chairman of the Department of Urology, Senior Director of Clinical Affairs for the Sidney Kimmel Comprehensive Cancer Center, and the Enterprise Vice President of Urology at the Jefferson Health System at Thomas Jefferson University in Philadelphia, Pennsylvania. Dr. Gomella's lab, a part of the Jefferson Sidney's Kimmel Comprehensive Cancer Center, is involved in clinical research for the development of techniques to treat cancers.