The Role of FSH in Prostate Cancer
How to cite: Garnick, Marc B. “Point Counterpoint: The Role of FSH in Prostate Cancer” January 27, 2018. Accessed May 2025. https://grandroundsinurology.com/The-Role-of-FSH-in-Prostate-Cancer/
(Twitter Question) Reveal the Answer to Audience Response Question #1
- True
- False
Reveal the Answer to Audience Response Question #2
- True
- False
Reveal the Answer to Audience Response Question #3
- A. Prostate cancer
- B. Breast cancer
- C. Glioblastoma,
- D. A and B only
- E. All of the above
Summary:
Marc B. Garnick, MD, discusses whether or not follicle stimulating hormone (FSH) affects prostate cancer progression and causes adverse effects to androgen deprivation therapy (ADT). He presents evidence for both the pro and con sides of this argument.
Point Counterpoint: The Role of FSH in Prostate Cancer – Transcript
Click on slide to expand
Role of FSH in Prostate Cancer and ADT Adverse Events—Is it important?
So to get to both sides of the argument about a follicle stimulating hormone, I’m going to discuss its components in prostate cancer and actually its emerging role in modulating and possibly causing some of these side effects of androgen deprivation therapy, and rather than taking a pro and con I’ve asked the question is it important. And I’ll leave the audience to make that determination at the end of the data presentation.
Disclosures
Okay, so those are my disclosures. I’m involved in two publications from Harvard Medical School. The one on the right is an annual report for which I receive an honorarium and serve as the editor in chief, and the one on the left is a brand new publication that some people in the audience may be interested in. This is a chronological evaluation of approximately 20 to 25 patients that we’ve cared for over the past 12 to 15 years in terms of seeing how they made determinations about their management of prostate cancer and whether they were happy with their management of prostate cancer and whether they were happy or sad with it, and that publication is coming out in several months. And I also receive an honorarium for serving on that. And I also serve as a consultant to Bayer on a DSMB.
Historical Overview of FSH
So I just want to provide a historical overview of FSH. Its effects on cancer cell lines in prostate and breast have been known really since the 1990s. Dr. Ben Joseph really did some very important work when he was at Wayne State. There is significant kinetic differences and varying forms of androgen deprivation therapy, and I’ll talk about the levels of FSH that occur following medical castration versus surgical castration, and it’s now being implicated in many of the adverse events that are associated with androgen deprivation therapy, and there’s been a renewed interest in the understanding of its biology now that we have the ability to modulate levels of FSH that can be achieved with GNRH antagonists. I think it’s that last bullet that has basically said, are there any differences between various forms of therapy on prostate cancer outcomes based upon looking at levels of FSH.
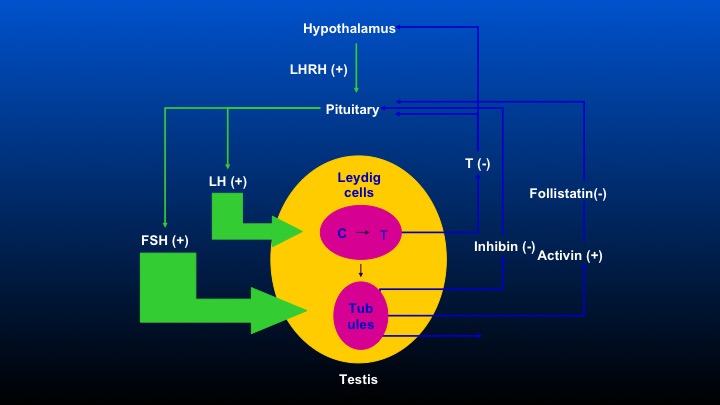
Hypothalamus
Okay. This is the comment that Dr. Partin was making. We have the hypothalamus, which stimulates LHRH, causes the pituitary to release LeHigh and FSH, and there are feedback loops including inhibin, activin, and follistatin that occur, so obviously, when one removes the testicle, there’s no feedback inhibition on the pituitary, and FSH levels following bilateral orchiectomy are really quite substantial, and the differences in FSH following either agonist or antagonist therapy will be a focus of this discussion.
Effect on FSH Levels of Current Hormonal Therapies
Okay, so here are the FSH levels on current hormonal therapies. Following bilateral orchiectomy, FSH levels increase substantially. Diethylstilbestrol, very interesting, causes a decrease in FSH levels, and I was just speaking to Dr. Glode at the break, and he’s got some very interesting data on the use of DES following all sorts of hormonal therapies that cause important tumor regression and PSA responses in his patient population. One wonders whether that’s potentially related to FSH.
LHRH agonists initially cause an increase in FSH followed by a decline, and then both with combined androgen blockade as well as with LHRH agonist, there is an escape in which FSH levels sort of come back to normal. Unlike the agonist, GnRH antagonists such as degarelix or abarelix causes a permanent, sudden, and precipitous decrease in FSH without going through the stimulatory followed by the decline. It declines really from day one.
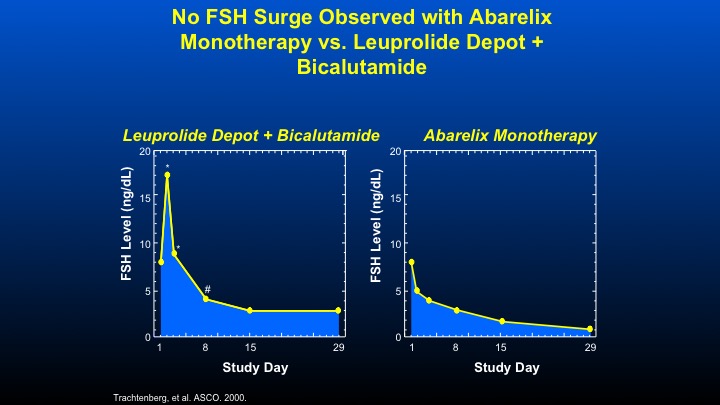
No FSH Surge Observed with Abarelix Monotherapy vs. Leuprolide Depot + Bicalutamide
Here are some data that we did with the initial GnRH antagonist Abarelix, looking at the combination of Leuprolide plus Bicalutamide looking at FSH levels in men with prostate cancer. You can see the increased peak, then a decline in contrast, a GnRH antagonist such as Abarelix, and the data—these data are virtually identical for Degarelix, causes an immediate decline in FSH therapy.
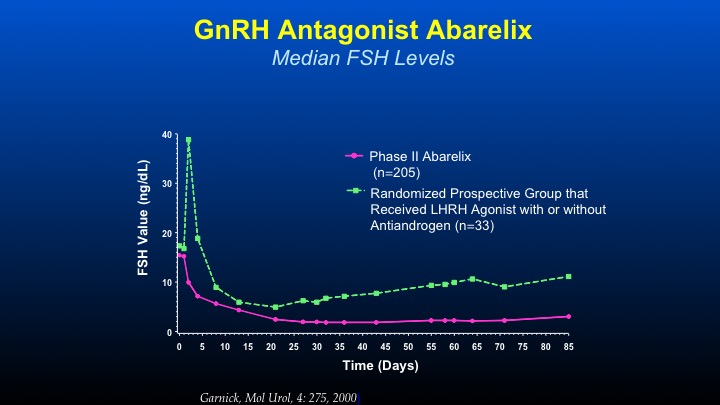
GnRH Antagonist Abarelix
The thing that we became very interested in—this was the first time with long-acting depot formulations of GnRH antagonists in which we could actually see chronologically the difference in FSH levels between—in the green line LHRH agonist with or without anti-androgen with the initial spike, decline, and then a gradual increase in contrast to GnRH antagonists causing an immediate decline, which is sustained during the period of therapy. And this was the first time that an agent was able to actually persistently and consistently cause a decline to levels not undetectable but low—very, very low levels of FSH, which had previously been unachievable. The very interesting thing, is even in patients who are on LHRH agonist therapy, if you orchiectomize them, the FSH levels continue to go up. So it’s a very important axis that’s maintained.
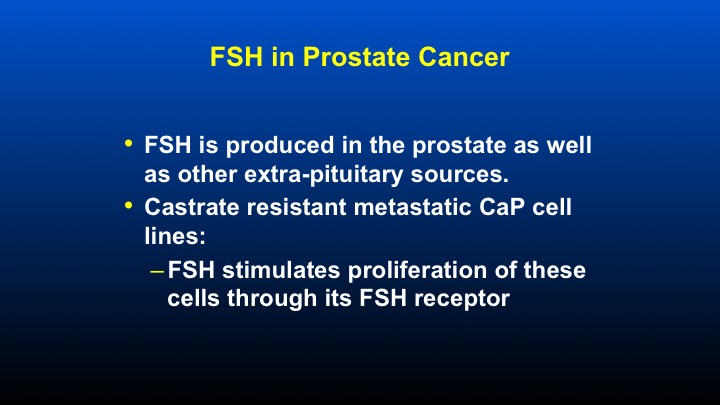
FSH in Prostate Cancer
So in prostate cancer, FSH is produced in the prostate as well as other extra pituitary sources. And castrate resistant metastatic prostate cancer cell lines, when exposed to FSH, causes a stimulation of these cells through its FSH receptor.
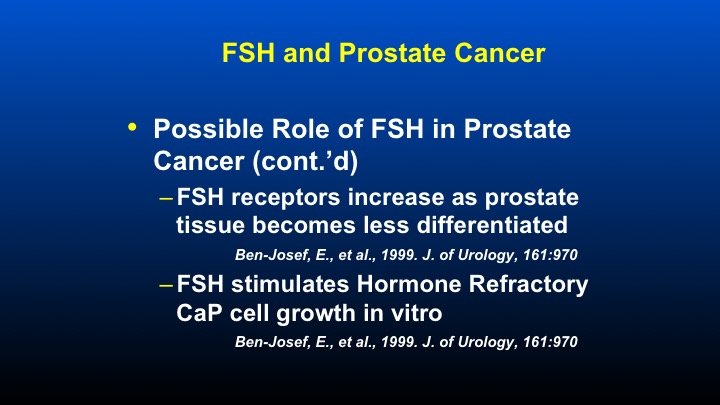
FSH and Prostate Cancer
Other aspects that have been looked at, as I mentioned, by Dr. Ben Joseph, who identified the potential role of FSH in prostate cancer that he mentioned or he showed that FSH receptors increase as prostate tissues became less differentiated, and FSH stimulates hormonally refractory prostate cancer cell growth in vitro. So these data have been known for quite some time, and we just didn’t have any really way of modulating or affecting levels of FSH prior to the identification of the effect that antagonists had.
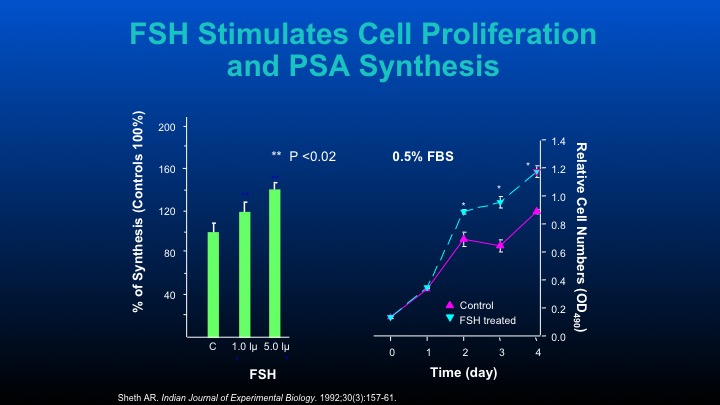
FSH Stimulates Cell Proliferation and PSA Synthesis
Those are some of the cell data that other people have performed.
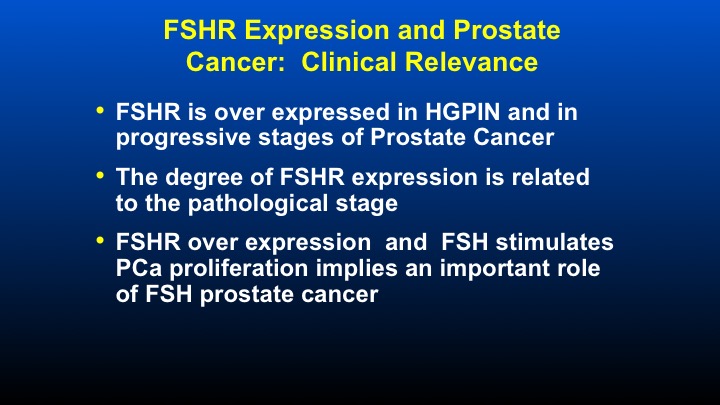
FSHR Expression and Prostate Cancer: Clinical Relevance
In terms of the FSH receptor expression and prostate cancer, the clinical relevance is also seen in tissue samples. FSH receptor is overexpressed in HGPIN and progressive stages of prostate cancer. Receptor expression is related to the pathological stage. And receptor overexpression and FSH stimulates prostate cancer proliferation and really implies an important role at least at the local level of FSH’s effect on prostate cancer and prostate glandular biology.
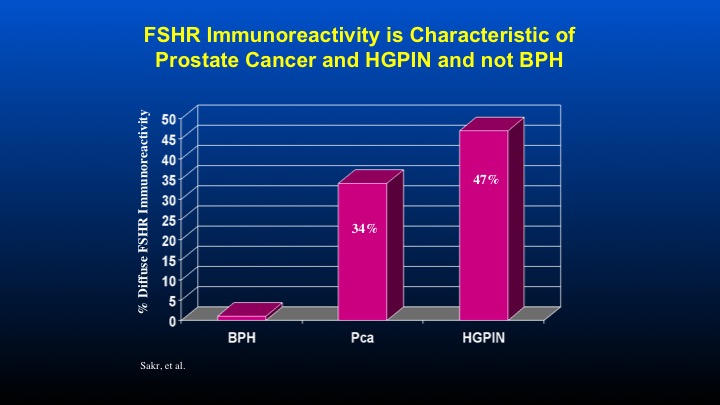
FSHR Immunoreactivity is Characteristic of Prostate Cancer and HGPIN and not BPH
Here are some data form Dr. Sakr looking at FSH receptor immunoreactivity compared to patients with HGPIN, as well as prostate cancer.
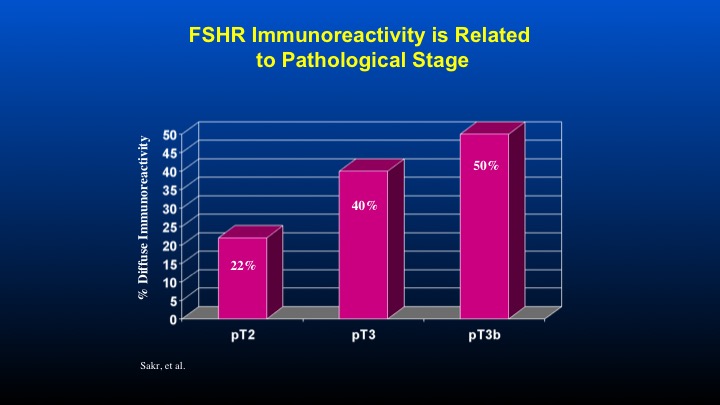
FSHR Immunoreactivity is Related to Pathological Stage
And if one takes a look at the T stage associated with FSH receptor immunoreactivity, again, a direct correlation with increased receptor reactivity as a function of the T stage of the prostate cancer.
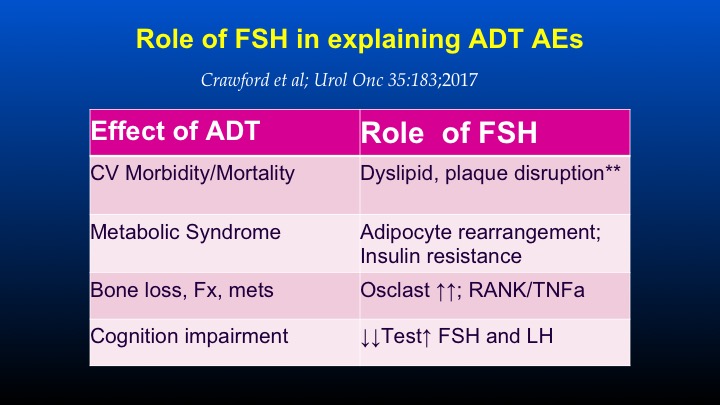
Role of FSH in explaining ADT AEs
So those are sort of the pre-clinical and in vitro models that suggested a role for FSH. I just want to change before we go to the human data on looking at the FSH as a possible explanation for some of the androgen deprivation therapy adverse events, and this was taken from a paper that Dr. Crawford organized with Dr. Marshall and Dr. Shally that I was able to participate in, basically a round table of people very involved in FSH really looking at the world’s literature of effects of FSH and its potential role in modulating some of these side effects on ADT, and there’s the citation just under the title. So, if one takes a look at the effect of androgen deprivation therapy, which will be the subject of subsequent talks on cardiovascular morbidity and mortality, FSH has a clear cut role in dyslipidemia, plaque disruption, and plaque formation.
So whether or not the role of FSH is potentially being modulated as a means of affecting cardiovascular morbidity and mortality is certainly out there, and I think there will be other discussions tomorrow on cardiovascular roles of androgen deprivation therapy.
In terms of the metabolic syndrome that can occur following ADT, FSH has a definite role in adipocyte rearrangement causing insulin resistance. In terms of bone loss, fractures and metastatic disease, FSH causes an increase in osteoclast activity, and that’s mediated through RANK ligand and TNF alpha. And in terms of cognition impairment, this is sort of an emerging role in which the role of ADT leading to decreases in testosterone and FSH may have some role in cognition and cognitive impairment.
So the whole concept of is it just low testosterone that’s leading to these abnormalities, or is low testosterone as a result of ADT being modulated through some of the actions of the multitude of actions of FSH.
FSH Has Been Shown to Play a Role in Breast Cancer
In terms of other cancers, FSH has been shown to have a role in breast cancer. Breast cancer cell lines show de novo FSH synthesis in breast tissue. There’s a very interesting correlation between patients with premenopausal breast cancer in terms of their ambient levels of circulating FSH not in post-menopausal women in which the FSH levels are high that basically correlate to the incident of breast cancer. Very interesting although agonist therapy is sometimes used in breast cancer with a 41% response in patients receiving goserelin, FSH occurs in these patients as would be expected, and Tamoxifen can prevent this FSH escape and also be associated with clinically meaningful responses. So there’s just a lot of information relating to the potential role of FSH in breast cancer, as well as in ovarian cancer where receptors are present in ovarian cells and FSH stimulates in vitro ovarian cancer cell growth.
FSH Has Been Shown to Play a Role in Ovarian Cancers
LHRH agonist therapy can inhibit the growth of some ovarian cancer cell lines, and agonist therapy can lead to partial remission and stable disease in advanced ovarian cancer patients, usually at the same time that the FSH levels have actually fallen from their peak levels.
FSH in Glioblastoma Stem Cells
And the final thing that I wanted to mention in terms of sort of the very interesting pre-clinical goes back to the question, this is a paper just published looking at the growth of glioblastoma multiforme. It turns out that so-called brain tumor stem cells are causing the recurrence of glioblastomas, and these stem cells are affected by epigenetic factors including a group of regulators that are called the ING regulators. These ING regulators, of which there are five or six, are actually inhibitors of a growth family of epigenetic regulators that work through histone acetylase and histone deacetylase complexes.
So it’s a novel group of growth family regulators that seem to be involved in causing stemness in patients with glioblastoma, and the ING requires activation of calcium channels and FSH pathway genes to increase the effect of increasing the actual number of brain cancer stem cells. So it’s a completely novel way of looking at tumor repopulation, especially in a localized place such as the brain in which FSH may have some role. And the citation of this paper was just published within the last 10 days.
Human Experience
What’s the human experience that can help try to shed some light? Again, we have not done as much as we should on this, but let me share with you one study that we were involved with several years ago looking at the role of potentially modulating FSH in two populations of patients. These were men at that time we called them hormonally refractory men. These are men who they would call today castrate resistant metastatic prostate cancer, and these data were done by Thomas Beer at the University of Oregon.
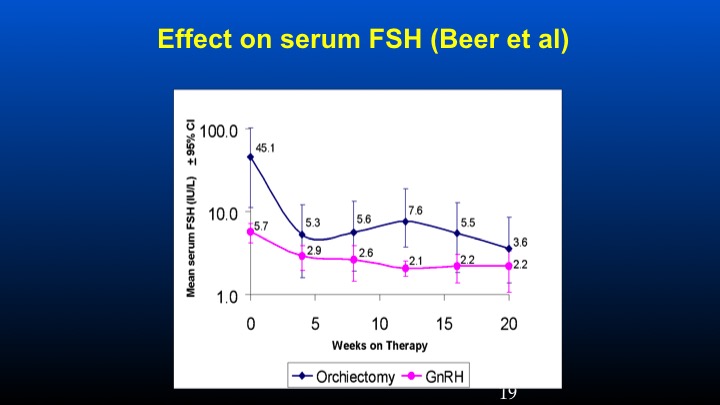
Effect on serum FSH (Beer et al)
The study was basically looking at patients who had previous orchiectomy or GnRH therapy in which their ambient levels of FSH were identified at presentation. These patients were then treated with conventional doses of GnRH antagonist degarelix, and we can see that the levels of FSH is really sky high in the orchiectomy patients at the time of entry to that study at levels of 45 compared to 5 to 6 IU/L of FSH in the 5 to 6 level in the LHRH-treated population. We can see that there was a dramatic and prompt reduction of FSH following the GnRH antagonist in the orchidectomized versus the GnRH previously treated patients.
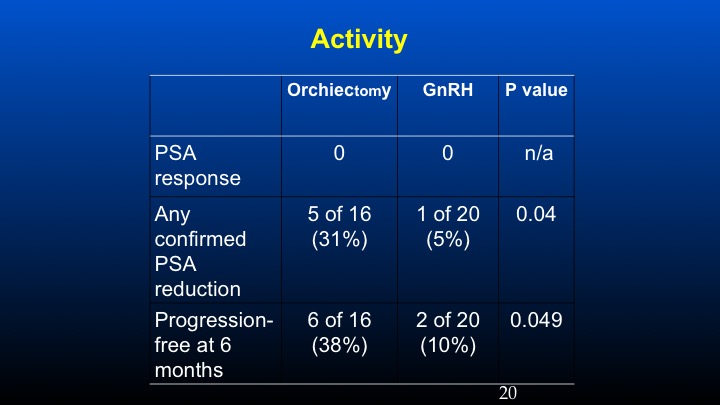
Activity
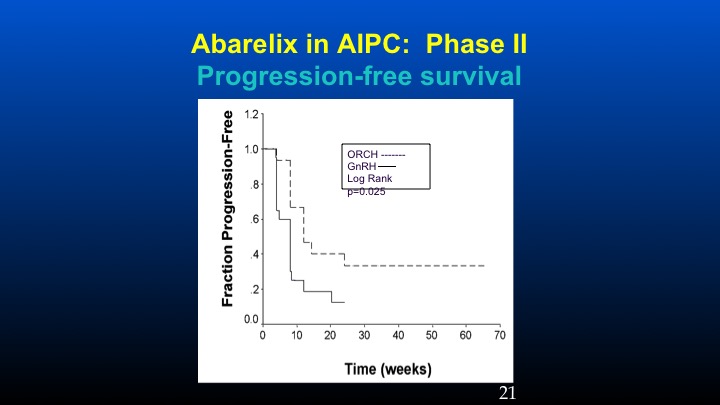
And if one takes a look at PSA response using conventional criteria, in the prior orchidectomized patients we had a 31% response rate compared to 5% in the LHRH population, and the progression-free survival at six months was basically 38% versus 10%. These data have been sort of looked at in other populations especially with degarelix, so there’s some information to suggest that in patients with high levels of ambient FSH following the development of castrate resistant prostate cancer, further reduction of FSH may play a potential role in further affecting some biochemical remissions.
Abarelix in AIPC: Phase II
And here’s the progression-free survival in the orchidectomized population versus the GnRH population.
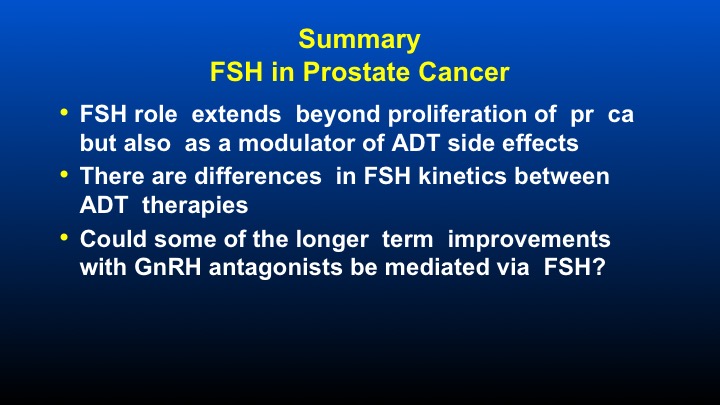
Summary FSH in Prostate Cancer
So I think in summary what I’ve tried to do is provide a very balanced view of the role of FSH, both pros and cons. It’s been around a long time. We finally have something that can modulate its levels. So I think in summary, FSH role extends beyond proliferation of prostate cancer, but also as a potentially very important modulator of androgen deprivation side effects. There are differences in FSH kinetics between varying androgen deprivation therapies. And could some longer term improvements with GnRH antagonists be mediated via FSH? So with that, I thank you very much for your attention and look forward to the discussion.
Marc, one of the things that I like out of this, we did this study a long time ago in SWOG of Lupron versus Flutamide, which was a six-month survival benefit, and then we did this other one, orchiectomy and Flutamide, and it didn’t show the same advantage. And so people initially said that Lupron is sort of a bad drug. Flutamide made it look good, and then flare and all of the other stuff—you didn’t get the flare with orchiectomy, and now we have FSH, so high levels of FSH. Do you think that explained the difference? That’s my first question.
The second is- why don’t we have some antibodies or something specifically against FSH? Because, you didn’t mention this but there are studies with GI tumors where people were given degarelix and they responded, stuff like that.
Dr. Garnick’s Answer:
So the first question has been a very perplexing question in terms of why the difference in survival between Lupron versus Flutamide and no difference in the orchiectomy study. FSH could certainly potentially be an explanation relating to that, but there is also the whole issue of the initial testosterone surge that occurs with LHRH analogs and whether or not that caused some disease worsening. If you take a look at those curves, they are basically the curves splay at three months. So all of the “badness” between the different therapies they really occur quite early. So whether or not a big huge bolus of FSH caused by the agonist could have caused that, whether or not the testosterone surge could have caused that is still unknown.
In terms of the FSH antibodies, you are absolutely correct. There have been some very provocative studies of GnRH antagonist, specifically degarelix being utilized in some non-prostate cancer studies, and I think it’s a very important target. It should be relatively easy to make a receptor binder for either the receptor or antibody to the molecule itself. So it’s an area of active investigation.
Question from Dr. Alan Partin:
My scientific mentor, Don Coffee, who unfortunately passed away this past November would have found this talk intriguing because he always said if this is true, what does it mean. Why would a metastatic or castrate resistant prostate cancer cell have a survival advantage to up regulating its FSH receptor?
Dr. Garnick’s Answer:
Yeah. So I mean I think you would have to basically take a look at the genomic profile of the genes involved in cellular proliferation in a metastatic castrate-resistant prostate cancer cell line to try to get more precision to answer that very, very important question, Alan.
Question from Dr. Lauren Klotz, MD:
Just a comment and question regarding Dave’s comment about why the initial SWOG Lupron trial was positive and the orchiectomy trial negative. That conversations been going on for years, but the Lupron trial was daily Lupron undoubtedly with poor compliance with the daily subcutaneous injection. So these guys’ testosterone was presumably bouncing all over the map, and to me that’s a far more plausible explanation for the benefit of the anti-androgen, which was also shown in the Marodi paper in patients whose testosterone failed to suppress compared to the FSH.
And my question is really along the same lines of how much of an impact the FSH is really having. Just as an example, orchidectomy is an effective therapy for prostate cancer, and maybe there’s subtle differences, but they’re not big differences in terms of its impact on the course of the disease. So how much—are we really kind of making a mountain out of a molehill? And a related question is if we’re looking at FSH, there’s other androgens and estrogens that are probably quite relevant as well, like estradiol we really haven’t paid much attention to some of these other potential hormones or intermediate androgens that may be playing a role.
Dr. Garnick’s Answer:
They’re two very important questions. The issue about orchiectomy being very effective therapy, no one would argue with that, but I’ve tried to find data on relative survivals and absolute survivals of orchiectomy-treated patients compared to hormonally treated, medically treated patients, and that data is very, very difficult to come by. The orchiectomy literature is very, very old. Those patients presented with far more massive disease than we would be treating today, but that’s a very important question. I don’t think there will ever be a study of orchiectomy versus medical therapy to try to help answer that question. So that’s an unknown question whether or not the orchiectomized patients died at a different rate compared to medically treated patients is totally conjectural right now.
ABOUT THE AUTHOR
Dr. Garnick is the Gorman Brothers Professor of Medicine at Harvard Medical School and the Beth Israel Deaconess Medical Center, where he also directs the hospital’s role as a tertiary cancer center for 7 affiliated community cancer centers. He has dedicated his career to the development of new therapies for prostate cancer. He serves as the Editor-in-Chief for The Annual Report on Prostate Diseases, formerly Perspectives on Prostate Disease. He served as the initial academic co-principal investigator for the commonly used prostate cancer drug leuprolide (Lupron). In addition to his academic affiliations, Dr. Garnick founded the Hershey Family Foundation for Prostate Cancer Research at Beth Israel Deaconess Medical Center, serves as medical adviser to World Book Encyclopedia, and is an active advisory member of several FDA panels and advisory committees. He has served on the boards of trustees of the Perelman School of Medicine at the University of Pennsylvania and of Penn Medicine. Dr. Garnick is also a Trustee Emeritus of Bowdoin College in Brunswick, Maine.