NET Background and Therapy
How to cite: Loaiza-Bonilla, Arturo “NET Background and Therapy” June 2, 2018. Accessed [date today]. https://grandroundsinurology.com/net-background-and-therapy/
NET Background and Therapy – Summary:
Arturo Loaiza-Bonilla, MD, MSEd, FACP, explains the basic scientific concepts behind neuroendocrine tumors (NETs), as well as classifying and staging these cancers. He then discusses the rationale behind targeting somatostatin receptors in NETs, therapy options, and future research in this setting.
An Introduction to NETs
Dr. Loaiza-Bonilla briefly describes the nature of NETs. Firstly, neuroendocrine cells are ubiquitous throughout the body. Therefore, epithelial neoplasms with predominant neuroendocrine differentiation can arise in most organs.
Although it is the traditional assumption that NETs are rare, in truth, their incidence and prevalence are increasing. Most of these cancers are slow-growing and malignant with metastatic potential. Furthermore, the incidence of gastropancreatic neuroendocrine tumors (GEP-NETs) is higher than that of most gastrointestinal (GI) tumors, with the exception of colorectal neoplasia.
Additionally, Dr. Loaiza-Bonilla discusses the breakdown of most common NET sites of origin according to geographical regions, the diversity of NET symptoms and presentations, the difference between NETs and carcinoid tumors, and the natural history of the disease.
Classifying NETs
Prior to 2010, the site of origin classifications for NETs were foregut, midgut, and hindgut. In 2010, the World Health Organization (WHO) then created a tumor-based classification system to replace those terms.
Dr. Loaiza-Bonilla defines the current NET site of origin categories as intestines, pancreas, and other. Also, he explains that “classification” refers to tumor characteristics and behavior, “grade” refers to inherent biological aggressiveness, measured by mitotic count and Ki-67, and “stage” refers to extent of the disease. It is important to realize that grade and stage are independent from each other.
Imaging for Staging NETs
The standard for staging these cancers involve either multiphasic computed tomography (CT) scans or MRI, depending on the location of the tumor. However, as physicians learn more about the behavior of NETs, novel positron emission tomography (PET)/CT imaging methods are proving to have increased specificity and sensitivity.
Treatment Goals
The treatment goals for NETs differ according to classification. Patients whose localized NETs are curative should undergo surgery, either with or without targeted techniques, with a curative treatment goal. The treatment goal for metastatic tumors is also curative surgery, if possible, along with targeted local and pharmacological therapies. For patients with asymptomatic, advanced, unresectable NETs, or for patients with small volume disease, observation alone is appropriate.
Therapy options for NETs
Traditional cytotoxic chemotherapies usually kill rapidly dividing cells by interfering with cell division throughout the body. Conversely, targeted therapies interfere only with specific cellular pathways necessary for tumor growth and progression. In this way, targeted therapies aim to fight cancer cells with more precision and potentially fewer side effects.
Future Directions
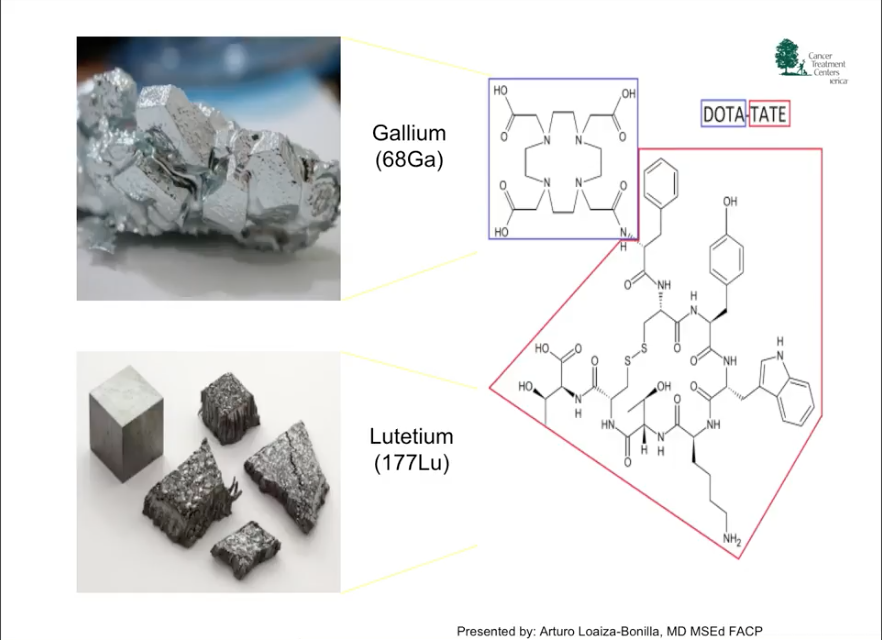
Dr. Loaiza-Bonilla claims that the future of management for NETs mainly involves combination therapies. Specifically, he summarized ongoing trials surrounding a PRRT/chemotherapy combination, or peptide receptor chemoradionuclide therapy (PRCRT), and an oncolytic virus treatment for NETs. Beyond new treatment options, he argues that future research in NETs should focus on combinations and timing of PRRT within the NET treatment paradigm.
ABOUT THE AUTHOR
Dr. Loaiza-Bonilla is Vice Chairman of the Department of Medical Oncology at Cancer Treatment Centers of America (CTCA) in Philadelphia, Pennsylvania. Dr. Loaiza-Bonilla is dedicated to comprehensive care for patients suffering from gastrointestinal malignancies, such as colon, esophagus, gastric, liver, pancreas, and neuroendocrine tumors. Dr. Loaiza-Bonilla earned a medical degree from the Universidad Nacional de Colombia in Bogotá, Colombia. Following medical school, he completed an Internship and Residency in Internal Medicine at Harbor Hospital Center in Baltimore, Maryland. Dr. Loaiza-Bonilla also completed a Fellowship in Hematology and Oncology at the University of Miami, Miller School of Medicine. Prior to joining CTCA, Dr. Loaiza-Bonilla was an Assistant Professor of Clinical Medicine at the Perelman School of Medicine at the University of Pennsylvania. During this time, he completed his master’s degree in medical education at the same university. Dr. Loaiza-Bonilla has been published in several medical journals and oncology publications. He is also a reviewer and counselor for several journals, special interest groups, panels and academic competitions. He has also served as principal investigator and co-investigator for many large oncology clinical trials. Dr. Loaiza-Bonilla has been a member and held leadership positions for several medical organizations. These include the Pennsylvania Society of Oncology and Hematology, the American Society of Hematology, the American Society of Clinical Oncology, and the American Association of Cancer Research.