New Treatment Options in LUTS
Matt T. Rosenberg, MD, presented “New Treatment Options in LUTS” during the 27th Annual Perspectives in Urology: Point Counterpoint on November 9, 2018 in Scottsdale, Arizona.
How to cite: Rosenberg, Matt T. “New Treatment Options in LUTS” November 9, 2018. Accessed Jul 2024. https://grandroundsinurology.com/new-treatment-options-in-luts/
New Treatment Options in LUTS – Summary:
Matt T. Rosenberg, MD, discusses treating of lower urinary tract symptoms (LUTS) through a framework of inhibiting overproduction of urine by the kidneys. In this dual-presentation, he describes behavioral modifications as well as pharmacologic agents for the management of overactive bladder (OAB) and nocturia.
To further expand your knowledge of the management of nocturia, visit the Nocturia Next Generation Learning Center.
Using a Broad Perspective of LUTS to Guide Treatment
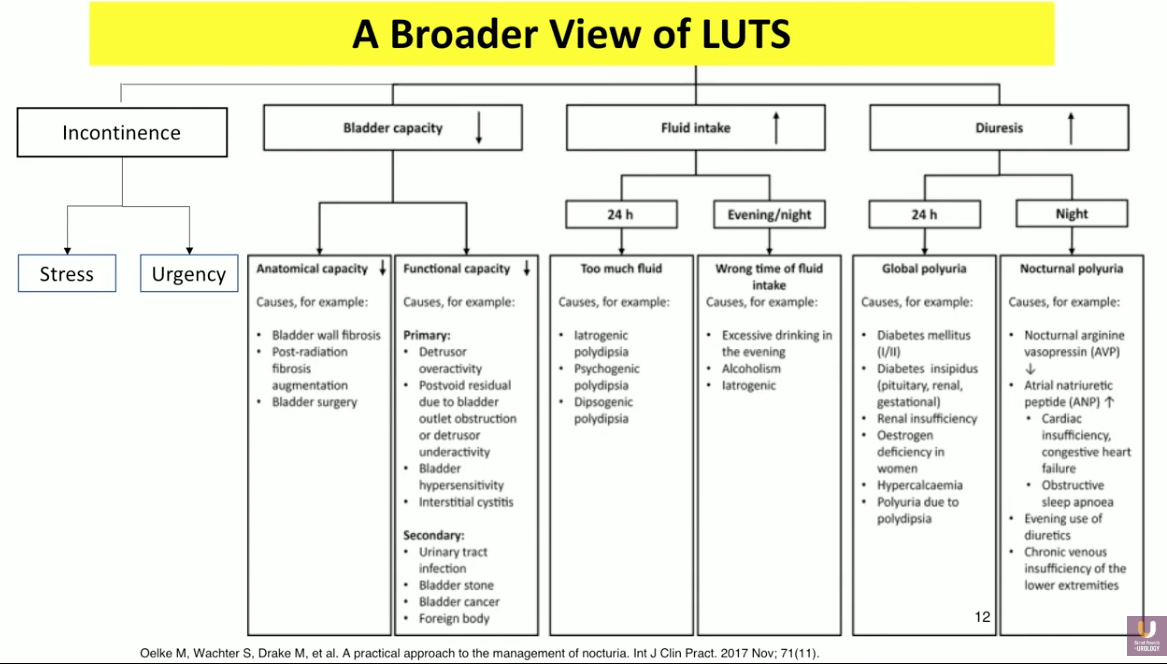
The definition of LUTs, according to the International Continence Society (ICS), is storage, voiding, and post micturition symptoms. The pathophysiology of LUTS includes incontinence, decreased bladder capacity, and increased fluid intake. In other words, the bladder’s inability to hold 300-500ml of fluid leads to OAB and incontinence. Benign prostatic hyperplasia (BPH) or urethral strictures can cause blockage of the urine outlet, also leading to leakage and incontinence. However, these definitions and aspects of LUTS focus on vehicles that hold urine rather than urine production. One cannot overlook increased diuresis, or the overproduction of urine by the kidneys, as a significant component of LUTS.
It is important to approach these symptoms as both independent and coexisting when considering management approaches. With this framework in mind, refractory disease may be due to the clinician attempting to treat the wrong disease.
OAB
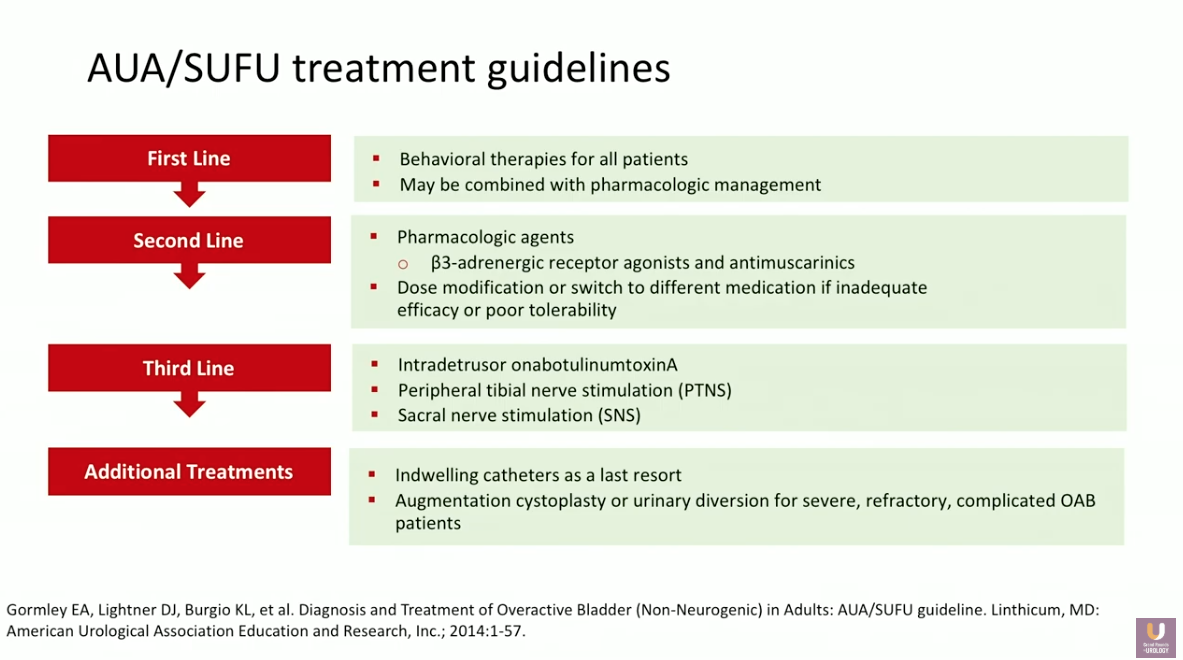
Returning to ICS definitions, OAB refers to “Urinary urgency, usually accompanied by frequency and nocturia, with or without urgency urinary incontinence, in the absence of UTI or other obvious pathology.” 29.8 million adults over the age of 40 experience OAB in the United States. The American Urological Association (AUA)/ Society of Urodynamics, Female Pelvic Medicine & Urogenital Reconstruction (SUFU) guidelines provide a framework for treating these patients, with first- through third-line options, as well as additional treatments for special occasions.
While clinicians should always consider behavioral modifications before other forms of intervention, this presentation focuses on second-line pharmacological agents.
New Indications for OAB
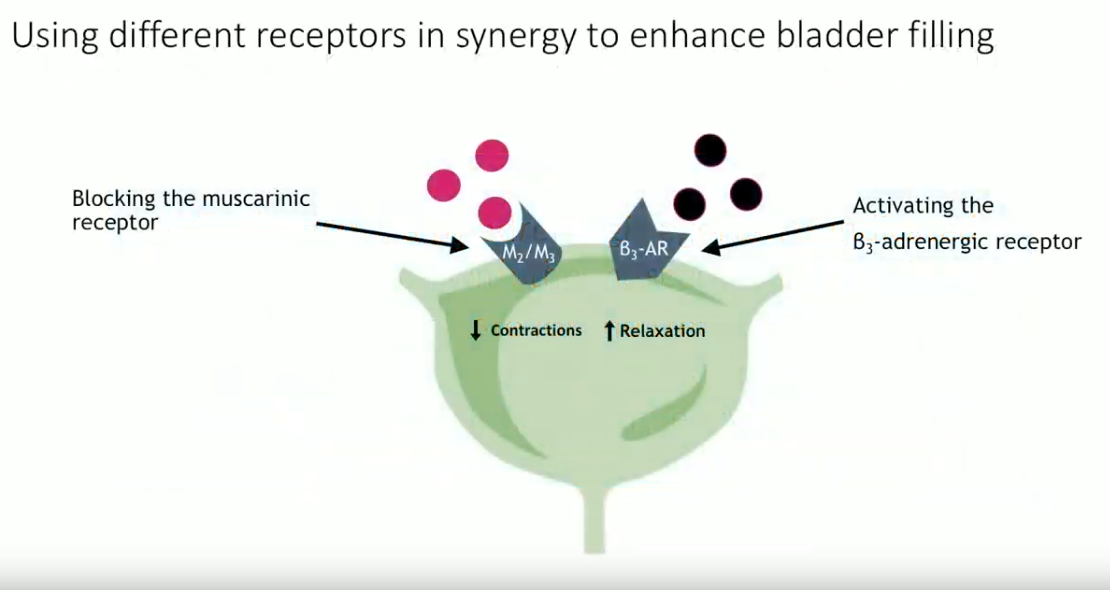
2018 marked a new indication for combination of mirabegron and solifenacin in refractory OAB. This combination blocks the muscarinic receptor to decrease bladder contractions. At the same time, it activates the β3-adrenergic receptor to increase muscle relaxation. Conclusions from the SYNERGY trial showed a significant improvement in urinary incontinence and micturition episodes per 24 hours with this combination compared to monotherapy. Notably, about 50% of patients had prior ineffective OAB medications at baseline.
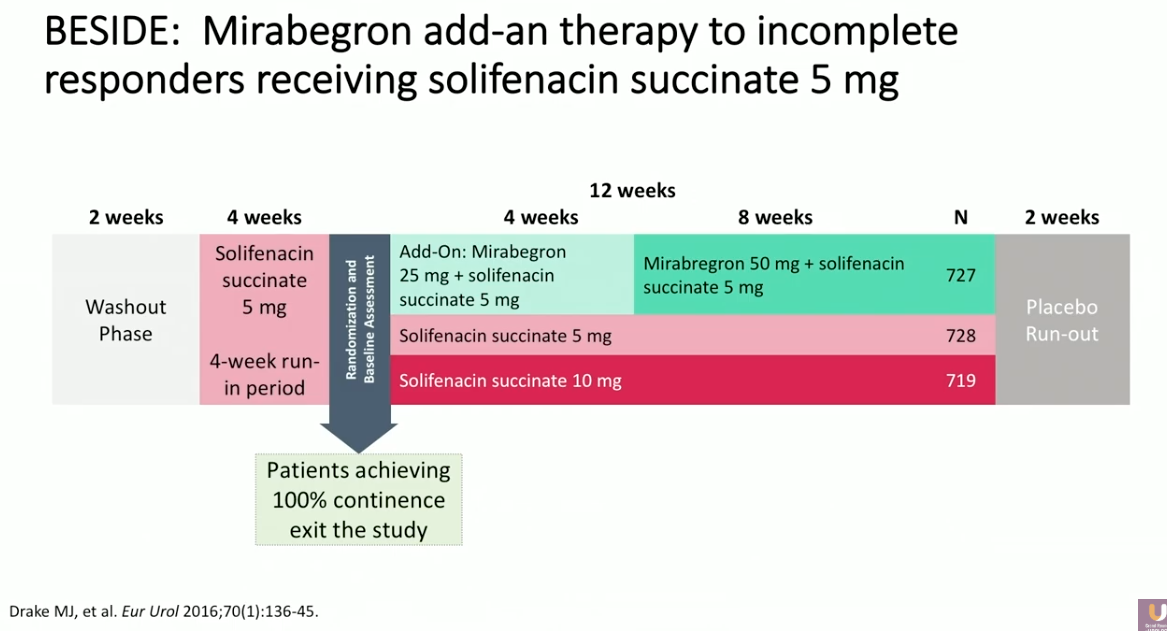
Although this combination has high efficacy, it may not always be appropriate to start a patient on this combination immediately. As an illustration of this, participants in the BESIDE trial received a low dose of solifenacin alone for a 4-week run-in period. The patients who achieved 100% continence after this period then exited the study. Only if patients did not completely respond at this time did they continue in the trial, either staying on the same therapy, receiving an escalated dose of solifenacin, or receiving a mirabegron/solifenacin combination.
While the combination had a relatively higher efficacy rate, it may be appropriate for patients who respond well to continue on monotherapy and lower doses. Dry mouth is the most common adverse event seen in this combination.
Nocturia
The ICS defines clinically meaningful nocturia as waking two times or more at night to void. Meanwhile, nocturnal polyuria is nighttime urine production more than 20% of the total urine production output in younger adults, and 33% of urine output in people over the age of older adults. However, a more accurate definition of nocturia could be considered the nighttime volume of urine exceeding the bladder capacity. By establishing this, urologists can better understand whether to approach treatment by increasing capacity or decreasing volume.
The Detriments of Nocturia and Sleep Loss
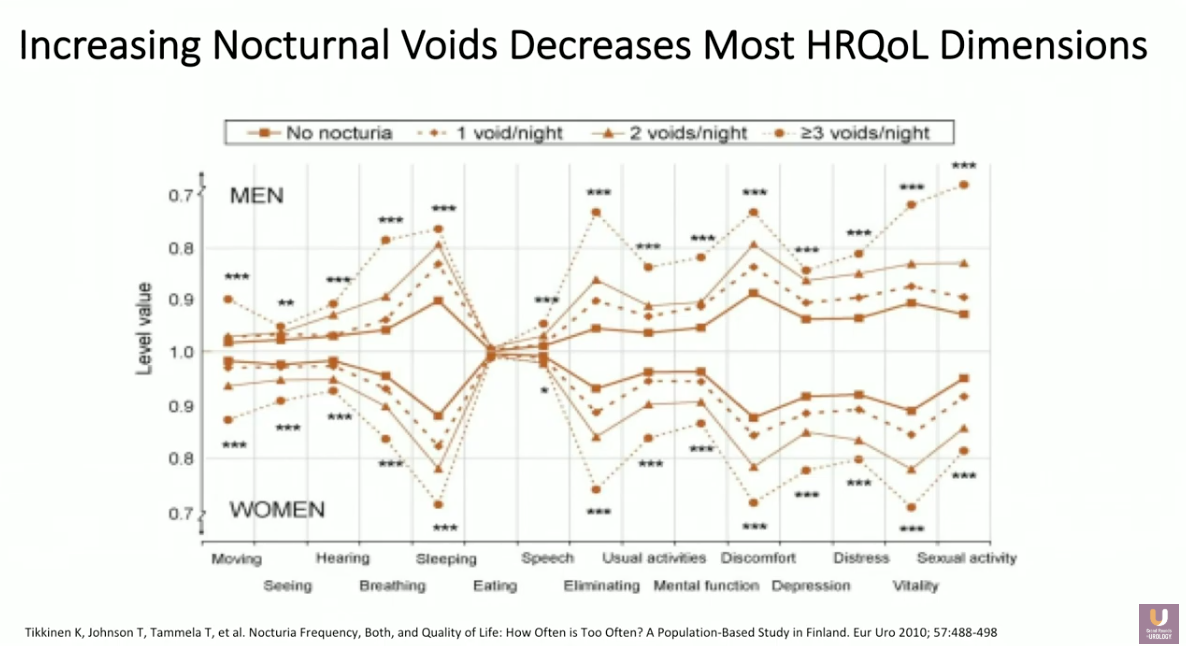
Nocturia and its corresponding sleep interruption have detrimental effects to quality of life. Significant adverse effects include reduced psychomotor performance, concentration, memory, and cognitive function in the short term. In the long term, consequences include depression, susceptibility to somatic disease, as well as risk of cardiovascular disease and car accidents. Data shows an association between nocturia-related loss of sleep and increased risk of fracture and death in elderly individuals, decreased health-related quality of life, and increased mortality risk.
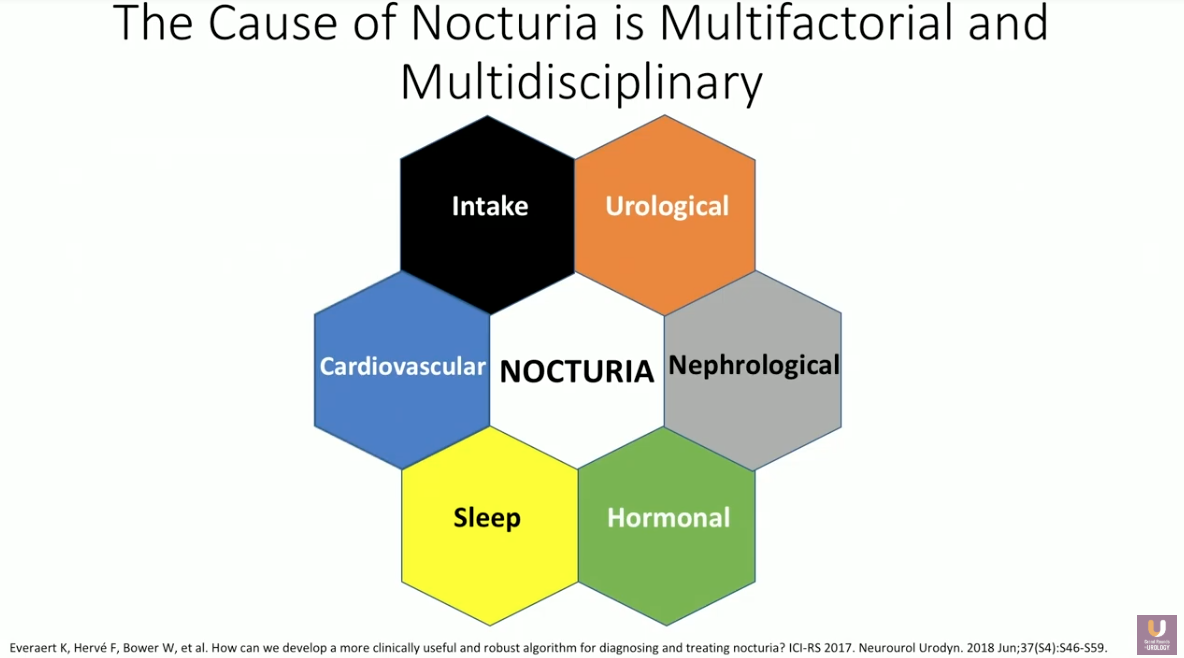
Though there are certain individual patient risk factors for nocturia, the cause of this condition is multifactorial and multidisciplinary. Because of this, a complete evaluation of nocturia must include a medical and surgical history, a focused physical examination, and assessment of urine and blood for infection or diabetes.
Behavioral Modifications
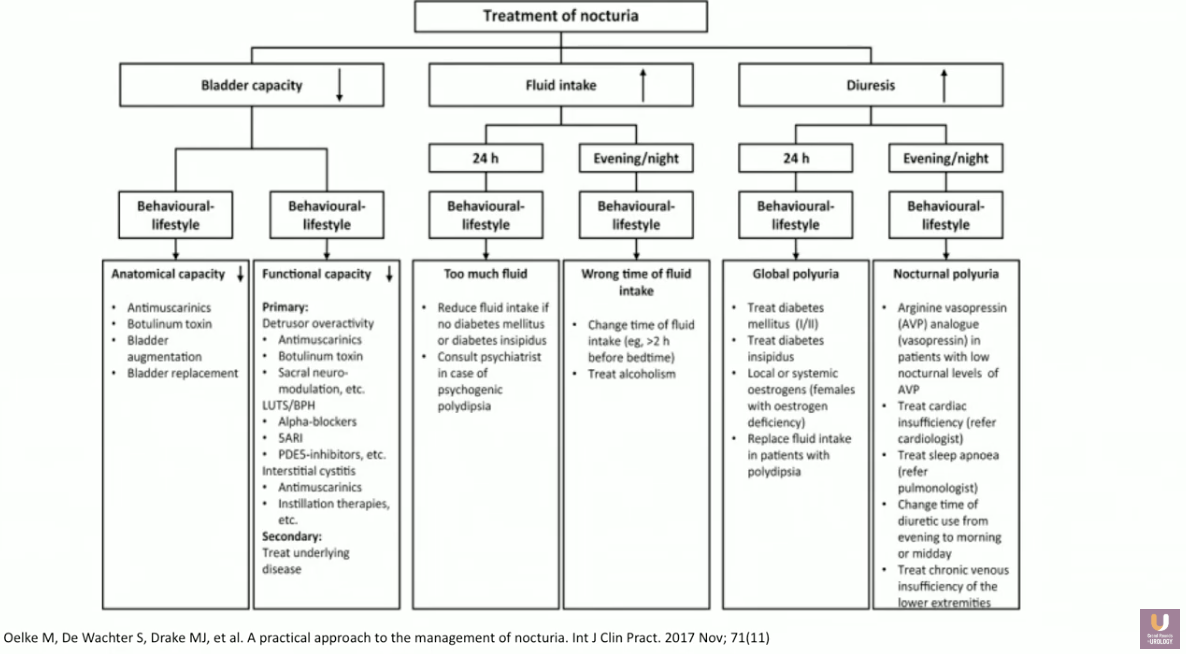
Despite the cause, clinicians should treat nocturia with behavioral modifications before moving to other forms of intervention. Simple behavioral modification considerations include decreasing nighttime and overall fluid intake, emptying the bladder before bed, as well as improving bladder hygiene. Some patients may benefit from reduction or avoidance of caffeine, alcohol, and salt. Counsel patients to alter the timing of diuretic medication administration from nighttime to morning. The use of compression stockings, leg elevation, as well as addressing sleep apnea, weight, and exercise, may also benefit certain patients.
Navigating Treatment Options for Nocturia
When nocturia persists despite behavioral modifications, individual symptoms should drive treatment. For instance, a poor stream may necessitate BPH-focused treatment. Antimuscarinics and β3 may be appropriate in cases of OAB. However, the majority of these patients have nocturia due to nocturnal polyuria.
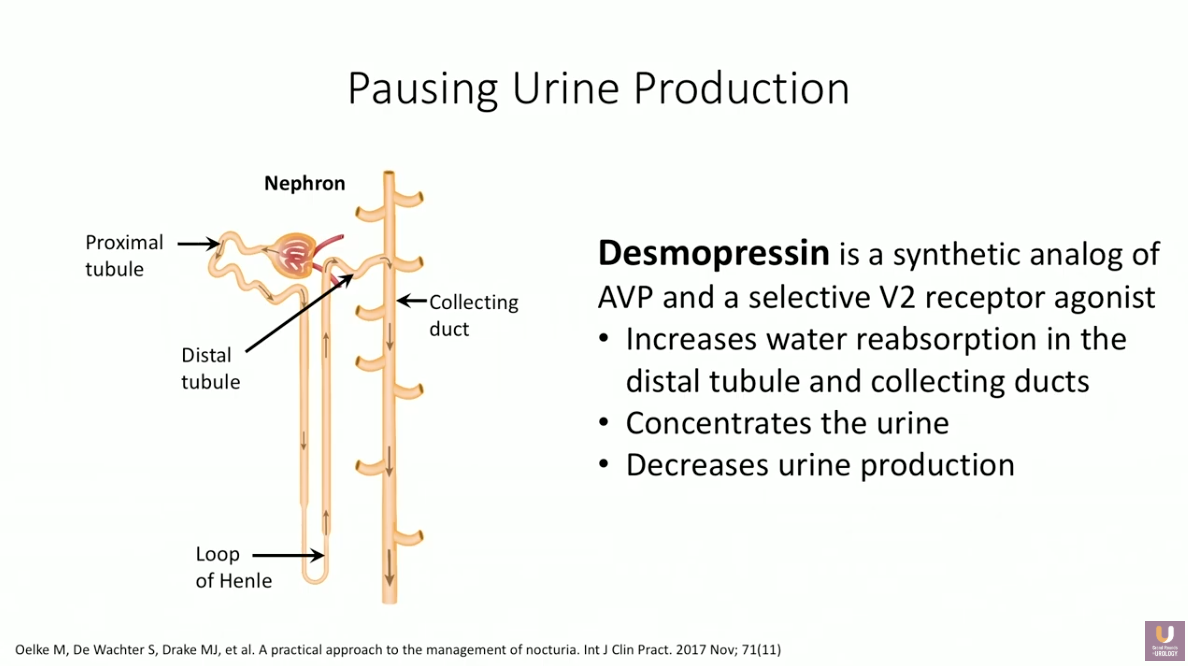
Though it is difficult to identify whether the etiology of nocturnal polyuria is overconsumption, overdiuresis, or lack of antidiuresis, treatment must focus on decreasing nighttime fluid production. Decreased production or potency of arginine vasopressin (AVP) inhibits reabsorption of water in the distal tubules and collecting duct of nephrons. Desmopressin is a synthetic analog of AVP and selective V2 receptor agonist that increases water reabsorption, therefore pausing urine production at night.
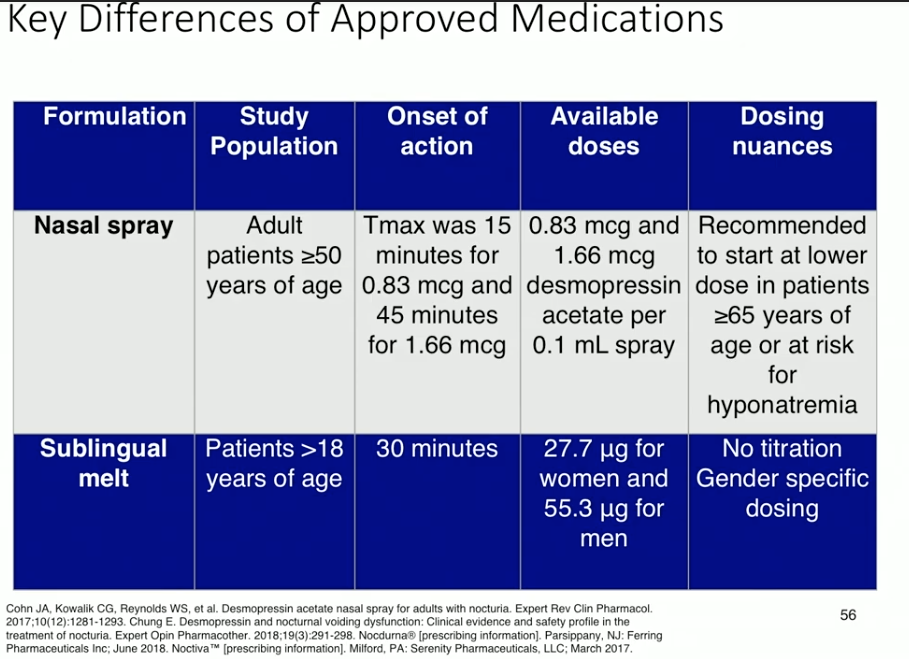
The currently available formulations of desmopressin for nocturia due to nocturnal polyuria are a nasal spray and a sublingual melt. In a study investigating titrated doses of sublingual desmopressin, increased dosage led to decreased nocturnal void volume. Placebo in this study also showed a response, which attests to the efficacy of behavioral modification. Sublingual desmopressin has shown to decrease number of nocturnal voids and extend the time to void in women and men. Data regarding the nasal spray shows similar efficacy results in nocturnal void volume, number of nocturnal voids, and time to void.
Special Populations: Nocturia plus OAB or BPH
For patients with simultaneous nocturia and BPH, low-dose oral desmopressin plus tamsulosin has proven to be efficacious and tolerable. Similarly, a combination of low-dose desmopressin and tolterodine proved to decrease nocturnal void volume and increase time to first nocturnal void in women with OAB and nocturnal polyuria.
Safety of Desmopressin
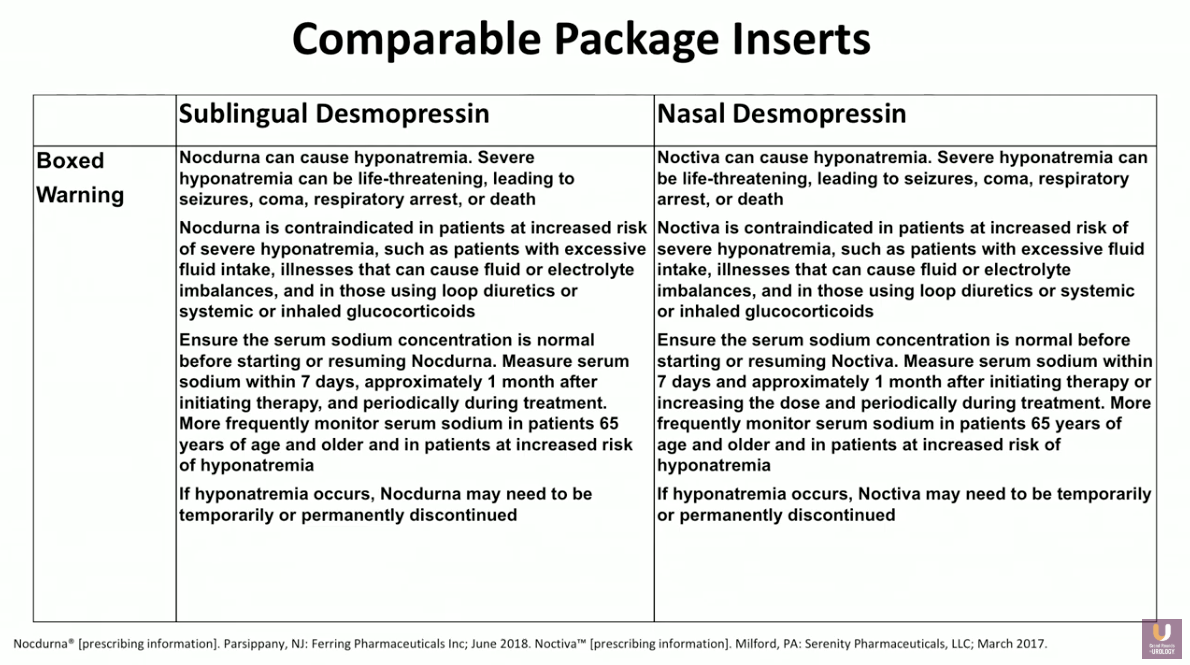
Formerly, the risk of hyponatremia has been the main concern with desmopressin. However, the duration of action in new forms of desmopressin allows for equilibration in the body, mitigating the risk. Regardless, it is important for clinicians to be aware of the risk factors for hyponatremia. Common risks including dose, age, as well as baseline serum sodium level. It is also important to monitor patient sodium levels prior to initiation and periodically during treatment.
Conclusions
In order to effectively evaluate and differentiate LUTS from other diseases, urologists and primary care physicians must work together. Additionally, the shared-care approach will allow safe treatment and monitoring of these patients.
About Perspectives in Urology: Point Counterpoint
Perspectives in Urology: Point Counterpoint (PCP) is an annual CME-accredited conference devoted to discussing and debating the latest topics in men’s health, general urology, and genitourinary cancers. The conference’s format includes not only didactic lectures, but also debates, point-counterpoint discussion panels, and unique-case presentations. Dr. Rosenberg presented this lecture during the 27th PCP in 2018. Please visit this page in order to register for future IUP meetings.
ABOUT THE AUTHOR
Matt T. Rosenberg, MD, earned his medical degree at the University of California, Irvine, where he trained in general surgery. He also trained in urologic surgery at Brigham and Women’s Hospital in Boston before changing fields to general practice. Dr Rosenberg has a special interest in the medical management of urologic diseases and has authored or coauthored articles appearing in Urology, The Journal of Urology, BJU International, The International Journal of Clinical Practice, and other peer-reviewed journals. He now practices in Jackson, Michigan, serving as Medical Director of Mid-Michigan Health Centers, and on staff at Allegiance Health, where he served as Chief of the Department of Family Medicine from 2003 to 2006. Dr. Rosenberg is a Senior Editor at the International Journal of Clinical Practice and is Founder and Chairman of the Urologic Health Foundation, a nonprofit group dedicated to the education of primary care physicians in the field of genitourinary disease. In 2011, he was appointed by the AUA Office of Education to be the Coordinator of Primary Care Education.