
PCa Commentary | Volume 174 – February 2023
Posted by Edward Weber | February 2023
MANAGING BIOCHEMICAL FAILURE AFTER RADIATION THERAPY — The Importance of Considering Both PSA and PSA Doubling Time.
The set-point for biochemical failure was standardized in 2006 based on a consensus conference of experts in Phoenix, Arizona. The value agreed upon was “PSA + 2 ng/mL above the post-radiation PSA nadir—hence the “Phoenix Definition” (Roach et al. Int. J. Radiation Oncology Biol. Phys. 2006). The intention was to set a standard definition to allow comparisons between clinical trials, different institutions, and different treatments—a value that correlated with cancer-specific and overall survival. They were careful to emphasize the difference between defining a definition applicable to individuals as opposed to a population, i.e., a clinical trial.
“It is very important for the readers to note that the definitions proposed are to define success or failure in the context of a population, not an individual. Defining PSA/biochemical success for an individual vs a population are separate questions with the former being guided by clinical judgment” … clinical judgment as guided by pretreatment PSA, Gleason score, tumor stage, and PSA doubling time (Roach, Ibid). One of the conference experts thought that including a measure of PSA dynamics, such as the slope of the PSA, would strengthen the metric, but this was thought too complicated for their purpose…
Phoenix Definition for Recurrence Is Inappropriate for Individual Management:
In today’s setting, two aspects of the Phoenix Definition may be considered weaknesses:
- It was constructed based on data obtained from imaging with the now-considered less sensitive CT and technetium bone scans, and
- a single PSA value falls short of accounting for the biological heterogeneity inherent in prostate cancer.
The deficiencies in the use of the Phoenix threshold to guide individual management was addressed by Jansen et al., Eur Assoc Urol. 2021 regarding detection of recurrent prostate cancer using PSMA PET scans in patients whose PSA values fell short of the Phoenix criterium after primary radiotherapy. Of 315 men studied, 63 were scanned below the Phoenix threshold. The Gleason score was 6 in 19%, 7 in 37%, 8 in 11% and 9-10 in 19% (unknown in 14%). Median PSA post-radiation nadir was <0.1 ng/mL and median PSA at the time of PET scanning was 1.3 ng/mL. The value for PSA doubling time was 6 months and time from PSA nadir to PET scan was 47 months.
**Findings: In 53 of the 63 scanned below the Phoenix threshold, 84.1% were PSMA-PET positive; 33% having a local recurrence at a single site, and 50.8% found to have metastatic spread. Their plea was for re-evaluation of the current diagnostic work-up for rising PSA after radiotherapy “to potentially improve patient selection for salvage or oligometastasis-directed treatment.”
Consideration for Individuals at Biochemical Recurrence.
This issue essentially is what PSA value at recurrence should prompt further evaluation, which further devolves to the question as to when a PSMA PET/CT is appropriate. Consideration of PET scanning involves two components: 1) What is the detection rate of the PET at different PSA levels, and 2) what is the likelihood at various PSA levels that a man’s cancer has spread and can be detected in locations such as within the remaining prostate or beyond.
**1) Detection Rate:
The detection rate at various PSA levels has been extensively reported for the Pylarify PET/CT (18F-DCFPyl-PET/CT) and the 68Ga-PSMA-11 PET/CT. The takeaway from a large body of data is that both scans perform well at low PSA values, values at which detection would lead to early treatment decisions, such as metastases directed stereotactic body radiotherapy. The detection rate for PSA values of <0.5 ng/mL is roughly 38 – 57%, between 0.5 and 1.0 ng/mL roughly 63 – 75%, and for PSA values between 1.0 – 2.0 ng/mL, 82-95%. The higher the Gleason score, the higher the detection rate.
**2) Predicting the likelihood that disease has spread at various PSA levels:
This issue was addressed by Pienta et. al in an article, “Predictors of 18-DCFPyL-PET/CT [Pylarify] Positivity in Patients with Biochemical Recurrence of Prostate Cancer after Local Therapy” Journal of Nuclear Medicine. December 2021. The purpose of their study was
“To investigate the factors predicting scan positivity and disease location in patients with biochemical relapsed (BCR) prostate cancer (PCa) after primary therapy using prostate-specific membrane antigen (PSMA)-targeted 18F-DCFPvL-PET/CT.”
The study group was predominantly comprised of men with high-risk cancer: Gleason score 7, 49%; and >8, 35%; with a median PSA value at PET scan of 1.6 and median PSA doubling time (PSAdt) of 6.8 months. All men were negative on conventional CT and bone scans and were not receiving ADT.
In the 50 men following prostate radiation the Pylarify scan was “always negative at PSA lower than 1.0 ng/mL and extra-pelvic disease was seen only when PSA >2.0 ng/mL.” The location of recurrence in 48% of men was intra-pelvic (75% within the prostate and 25% in pelvic nodes) and in 44% recurrence was beyond the pelvic. The predominant predictors for recurrence in this post-radiation cohort were PSA and the PSA doubling time leading up to the performance of the PET scan. “The PSA and PSAdt are able to predict scan positivity and disease location. In our cohort, at PSA >2.0 ng/mL, PSMA-PET was almost always positive for recurrence, with distant disease seen in about … 45% of [men] post-RT,” and was significantly higher for men with a rapid PSAdt. The nomogram presented below integrates PSA and PSAdt values.
Two examples based on the nomogram illustrate the importance of PSAdt on recurrence. The prediction for extra-pelvic recurrence is ~15% for a man whose PSA post radiation became 2 with a PSAdt of 15 months. For another man with PSA of 2 ng/mL whose PSAdt is 6 months the prediction for extra-pelvic recurrence is ~40%.
BOTTOM LINE:
At biochemical recurrence after primary irradiation, the combination of the PSA and PSA doubling time can determine the likelihood of detecting metastases with PSMA PET/CT.
This is the nomogram as appears in the Pienta article presented with permission of the author and the publisher. Based on only 50 patients, its performance will be strengthened with future validation with a larger database.
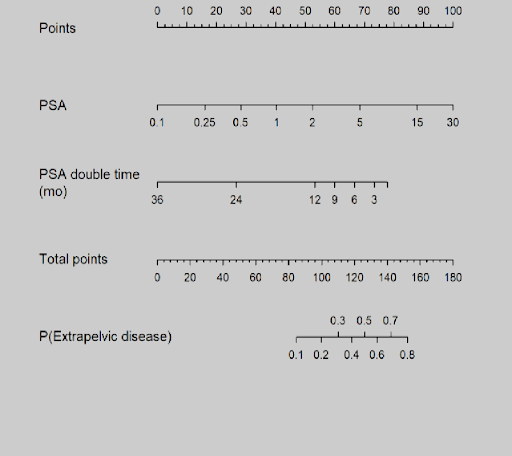
Nomogram for the radiation cohort
Instructions: locate patient’s PSA value and draw a straight line to the Points axis to determinate the amount of points towards the probability of a positive scan. Repeat this process for each variable and sum the points for each predictor. Locate the final sum of points on the Total-point axis and draw a line straight down to find the probability (P) of having a positive scan or having a scan with extra-pelvic lesions.
Your comments and requests for information on a specific topic are welcome e-mail ecweber@nwlink.com.
Please also visit https://prostatecancerfree.org/prostate-cancer-news for a selection of past issues of the PCa Commentary covering a variety of topics.
“I want to thank Dawn Scott, Staffperson, Tumor Institute Radiation Oncology Group, and Mike Scully, Librarian, Swedish Medical Center, for their unfailing, timely, and resourceful support of the Commentary project. Without their help this Commentary would not be possible.”
ABOUT THE AUTHOR
Edward Weber, MD, is a retired medical oncologist living in Seattle, Washington. He was born and raised in a suburb of Reading, Pennsylvania. After graduating from Princeton University in 1956 with a BA in History, Dr. Weber attended medical school at the University of Pennsylvania. His internship training took place at the University of Vermont in Burlington.
A tour of service as a Naval Flight Surgeon positioned him on Whidbey Island, Washington, and this introduction to the Pacific Northwest ultimately proved irresistible. Following naval service, he received postgraduate training in internal medicine in Philadelphia at the Pennsylvania Hospital and then pursued a fellowship in hematology and oncology at the University of Washington.
His career in medical oncology was at the Tumor Institute of the Swedish Hospital in Seattle where his practice focused largely on the treatment of patients experiencing lung, breast, colon, and genitourinary cancer and malignant lymphoma.
Toward the end of his career, he developed a particular concentration on the treatment of prostate cancer. Since retirement in 2002, he has authored the PCa Commentary, published by the Prostate Cancer Treatment Research Foundation, an analysis of new developments in the prostate cancer field with essays discussing and evaluating treatment management options in this disease. He is a regular speaker at various prostate cancer support groups around Seattle.



