R. Jonathan Henderson, MD, presented “The LUGPA Alternative Payment Model (APM) on Newly Diagnosed PCa and the Role of Active Surveillance” at the International Prostate Cancer Update on January 27, 2018 in Beaver Creek, Colorado.
How to cite: Henderson, R. Jonathan “The LUGPA Alternative Payment Model (APM) on Newly Diagnosed PCa and the Role of Active Surveillance” January 27, 2018. Accessed [date today]. https://grandroundsinurology.com/The-LUGPA-Alternative-Payment-Model/
Summary:
R. Jonathan Henderson, MD, summarizes the Large Urology Group Practice Association’s (LUGPA’s) efforts to develop an alternative payment model (APM) for urologists treating newly diagnosed prostate cancer patients. He explains the roadblocks to reaching this goal, including conflicts with the Physician-Focused Payment Model Technical Advisory Committee (PTAC).
The LUGPA Alternative Payment Model (APM) on Newly Diagnosed PCa and the Role of Active Surveillance – Transcript
Click on slide to expand
Disclosures
By way of disclosures, the top bullet are my disclosures. I have a speaking arrangement or consulting agreement with those companies, and this project itself was funded by LUGPA, Myriad Genetics, and Integra Connect. I want to thank Drs. Cooperberg, Davis, Penson, and Stimson, who are not LUGPA members, but helped tremendously in this project, and the statistical work and actuarial analysis was done by Milliman and Associates.
LUGPA APM Overview
The overview, you know, MACRA—and Dr. Shore explained this process a couple of days ago, but MACRA established two main paths for payment in the United States going forward, MIPS, merit-based incentive payment system, which is continued fee for service medicine, with some supposed quality measures put in. And then alternative payment model, which included two-sided risk where you basically have to say, look, if we’re putting, if we’re doing better quality medicine, we want to share in the savings that we’re providing, so you are basically putting your money where your mouth is. This past month though CMS followed up on MIPS, which they have watered down consistently over the past two years, and they have watered it down to the point where MIPS is basically no change from the past fee for service that we have lived under for the past 20 years. To qualify for an APM, you have to have 20% of your Medicare patients eligible and participating or 25% of your Medicare dollars at risk in that goal.
So Deepak Kapoor and I sat back, and we thought about it. What would be the easiest way to create an APM that would allow our groups to qualify and it seemed that prostate cancer would be the one code that universally would be most likely to capture 20% of our patients or 25% of our dollars.
Principals
So the principals, and my apologies to IMP that got transliterated there, but Dr. Kapoor’s group, IMP in New York is a large group, over 100 physicians, large metropolitan area, and they have ancillary services. My group in Shreveport, Louisiana has about 20 physicians, a small city and ancillary services, and after we started the project, we realized that we really need to look at somebody to make this attractive to all urologists who doesn’t have ancillary services, so we got with Todd Cohen‘s group in Charlotte, NC, which is 40 physicians, but they are a CON, a certificate of need state, so they own no ancillary services, and that actually proved well when we got to talking with CMS.
LUGPA APM Overview #2
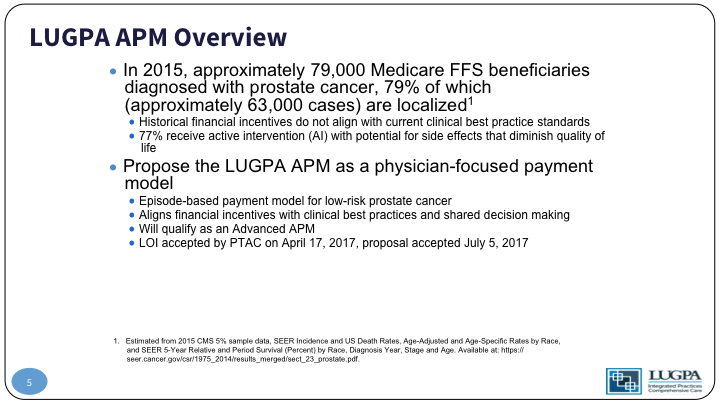
So, in overview of the background, we know that approximately 79,000 Medicare beneficiaries are diagnosed with prostate cancer each year, and about 63,000 of those are localized prostate cancer. We also have to recognize the historical fee for service does not align with current best practice standards. It doesn’t—well, it proves itself out in the fact that 77% of the patients receive active intervention. So, we proposed a physician-focused payment model that is episode-based payment for low-risk prostate cancer. It aligns financial incentives with clinical best practices, and most importantly shared decision making with the patient, qualifies under statute as an advanced APM. We submitted a letter of intent, which was accepted by PTAC. So you’ve got CMS, runs all of Medicare, and under CMS is CMMI, the Centers for Medicare and Medicaid Innovation, which runs these new projects. And under CMMI is the PTAC, the Physician’s Technical Advisory Committee that has to approve these new APMs. So, they accepted a letter of intent and the proposal was accepted in July.
LUGPA APM Overview #3
Then we went to Maryland and met with CMMI in July, had a lot of questions and answers, about two-hour session, was really good. They gave us some things we needed to work on. Went back to the drawing board, then submitted with the updates. It was reviewed by the PRT. Again, everything with the government is alphabet soup, right? The PRT is the preliminary review team, which is a subsidiary of PTAC. So the PRT reviewed it in October, had a few more suggestions, and questions back to the drawing board again, final proposal submitted and reviewed by PTAC this last month, and I will give you an update later on.
LUGPA APM Overview #4
So what this APM did, is it is—the episodes were attributed to urology practices. Dr. Shore went over the attribution process a couple of days ago, pretty complex and sort of silly, but in our APM, we guarantee that when you have a patient diagnosed at your institution with a prostate cancer, everything thereafter gets attributed to you because the total 12-month cost of care is attributed to you. I’ll go into why that matters a little bit later.
It is a two-part payment model. So once you have a patient who enters active surveillance, you receive a $75/month care coordination fee, and at the end of a 12-month period, there is a performance-based payment that basically the savings you have performed for the system in coordination with the quality metrics you have to hit gets shared between you and CMS.
So, the quality measures I’ll go into later include efficiency and cost reduction, communication and coordination, outcomes measurement and process. We use the oncology care model as a basis for our foundation, really, because it is one of the few APMs that’s been approved. It seems to work well for medical oncologists.
Beneficiary Population
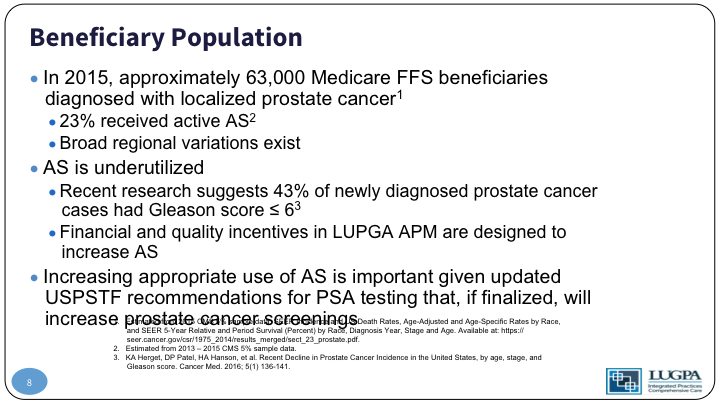
So, the population, again, 63,000 of the Medicare beneficiaries have localized prostate cancer. Only 23% in 2015 received active surveillance, and there is a huge variation across regions. If you look at the Medicare regions across the country, the variation active surveillance uptake went from about 19% to 25%, but if you drill down deeper into a county or metropolitan area level, that variation was from 10% to 70% utilization of active surveillance across the country. So, there’s a huge variation. We know it’s under utilized, and research shows that about 43% of localized prostate cancer has a Gleason 6. So, they should be candidates, and what we wanted to do was align the financial and quality incentives to increase utilization of active surveillance.
And we have to admit that the USPSTF recommendation was in part spurred by the fact that we weren’t utilizing active surveillance enough.
Proposed Episode Definition
So, the proposed definition for an episode is a 12-month period that begins the date of the diagnosis of prostate cancer. The patient then is attributed to that practice. I’ll show the active interventions on the next slide. Active surveillance is defined as no active intervention 12 months after a biopsy. So, it’s very simple, and the really nice thing is you don’t really have to do anything at the group level, because Medicare tracks all of this from the claims data that is already being submitted. So, the subsequent episodes for the beneficiaries, for 12 months you get the care management fee, and that care management fee is not included in the total cost.
Estimated National Episode Volume & Cost
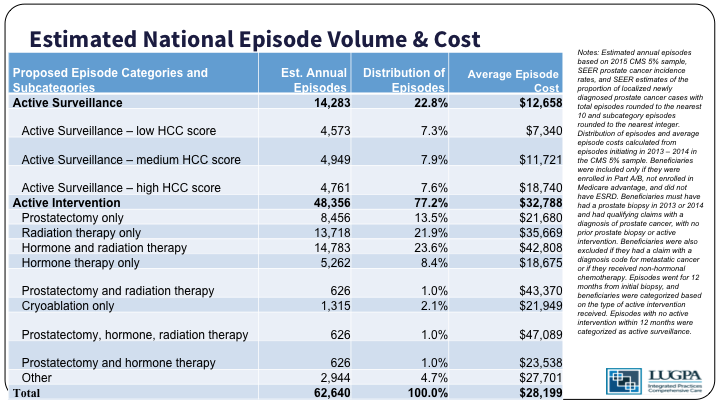
So, as you can see, active surveillance patients are then stratified by how sick they are into tertiles, and the active intervention basically is everything that you can think of goes into that bucket.
Estimated National Episode Volume & Cost #2
So, active intervention spending is $20,000 per-patient per-year higher than active surveillance in the initial 12 months. For those patients who would have received a prostatectomy, staying on active surveillance saves about $9,000 a year. For those who would have received radiation therapy, surveillance saves about $23,000 a year. And so, our model shows about a 250 million dollar savings per year for Medicare.
Care Management Fee
The care management fee, again, it’s $75 per-patient per-month. The rationale for that is active surveillance. Retention is very low. There’s a number of reasons for that, and so, what does this fee encourage? It encourages the provider to spend more time on beneficiary compliance with appointments, on education about disease progression, lab coordination, and most importantly, shared decision making with the patient, social service coordination, and then care revision, the plan revision, and review monthly.
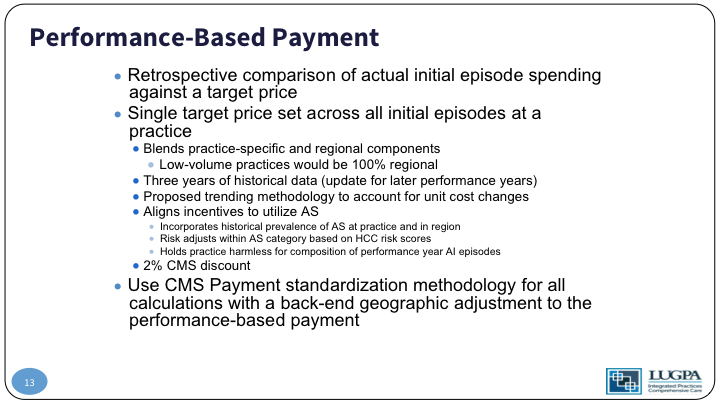
Performance-Based Payment
In addition to that monthly payment, we’re looking at a retrospective comparison of the actual spending episode over that 12-month period, along with a target price, and that target price is set across all initial episodes of practice based on your historical cost of care for that patient, and it blends your practice specific cost with the region’s cost. Low-volume practices who couldn’t have a statistically meaningful individual price set would be given 100% of the regional goal. So again, that is based on three years of historical data. To back up a little bit, when we started this project, we did a three-year retrospective chart review to look at cost analysis. We then convened a panel of experts and made an algorithm for active surveillance and did a prospective, six-month period using that algorithm to determine what practice cost would be.
So, we intend to align incentives to utilize active surveillance. We incorporate the historical prevalence of active surveillance at that practice and blend that with the regional. That gives us a target that we are, again, the shared performance-based payment after 12 months is going to be distributed on quality, and the delta of your historical active surveillance rate, along with your new active surveillance rate.
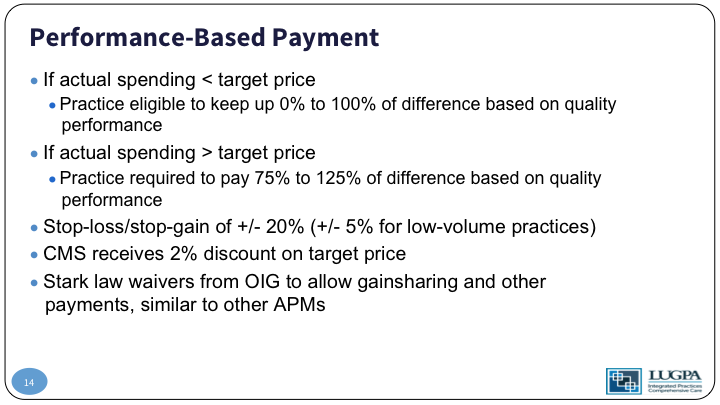
Performance-Based Payment #2
So, if your actual spending over that 12-month period is less than the target, your practice gets to keep up to 100% of the difference based on quality performance. If the actual spending is greater than the target price if you are more expensive than you should be, your practice is going to have to give money back to the system, up to 125% of the difference, again, based on quality measures. I’ll get to that in a minute. What’s important, though, is off the top, CMS takes 2% of the target price. Why that is important is CMS is guaranteed by that to save money. There’s some Stark law implications that have to be changed, and we won’t get into those weeds.
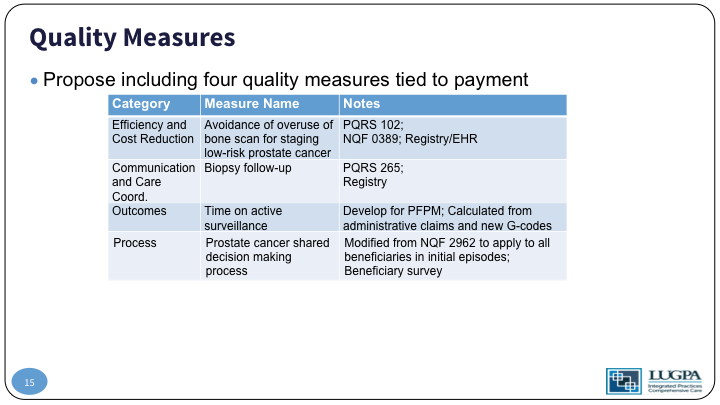
Quality Measures
So, the quality measures that we propose, number one on efficiency and cost reduction- there already exists a quality measure for the avoidance of over use of bone scans in low-risk prostate cancer. Communication care coordination- there already exists a code for biopsy follow-up with the patient outcomes is a new code. So, time on active surveillance- we would create a new G-code that would report that as a quality measure, basically how well are you doing keeping a patient on active surveillance, and then process, which is a shared decision making code, which is modified from an existing NQF code.
Time on Active Surveillance Quality Measure
So, time on active surveillance quality measure. Active surveillance is easily measured in claims data by no active intervention in a 12-month period. The G code is filed when a patient leaves active surveillance based on that patient’s choice, based on your determination that he is non-compliant or his disease has progressed. Again, no extra filling is needed. It’s calculated from claims data.
Shared Decision Making Quality Measure
So, the shared decision making quality measure is modified from 2962, and basically the patient just answers a questionnaire that says, “Did the provider describe the options between intervention or surveillance? Did he discuss the reasons for intervention or the reasons for surveillance? And most importantly, did the patient perceive that he was involved in that decision making process?”
So What Happened?
So what happened when we got to PRT? The preliminary review team voted against approval, but it still went forward to PTAC. After they vote against approval, we already had letters of support from the AUA and the AACU, but we then got some physician experts around the country help write letters of support. And also, the patient advocacy groups PHEN, ZERO, and PCEC also wrote letters of support. Nevertheless, we got to PTAC, and Dr. Shore and Kapoor presented the proposal there, they still voted 8:3 against approving the measure stating that it only met 8 of the 10 criteria, which is, I have to point out, far better than any other proposal thus far has gotten from that committee. They pointed out that ours is by far the best proposal they have received since MACRA passed. They disagreed in the 2 out of 10 criteria based on payment methodology. They did not like, philosophically, the fact that we would be attributed the total cost of care in that 12-month period. And care coordination, they did not like the fact that, per-patient per-month fee, we didn’t have an outline that showed a formula, basically, how everybody else who participated in the care of that patient would receive a portion of that fee. Basically, if you’ve got internists, how much are you going to share with them with that $75/month, and if you think about it, we couldn’t put a formula in there, because there is no way to formulate every locality in the country, and how they should distribute that. It should be left open.
PRT Comment
In their comments, it’s a little telling here, they said the majority of the PRT thought the proposal technically satisfied many of the high priority payment model criteria. The PRT members all do believe this proposal would align incentives more in the direction of the evolving standard of care, but we also unanimously felt the net effect of this proposal is wholly unsatisfactory in the signal its favorable recommendation would send to other applicants and specialties. So, in other words, make an example out of us. The PTAC process is unlikely to serve the secretary, the Medicare program, or the Country well if it comes to be seen as a device to be paid more for providing guideline-recommended care for patients. And at this point, we did a little AFLAC duck thing? Huh? Because that is what MACRA said you are supposed to do.
More PRT comment
Further comments from PRT, “It is difficult to estimate how long the transition to active surveillance for appropriate patients would take under the current payment system, as the shift requires changing behavioral patterns within a specialty.” Huh? That’s exactly what we’re trying to do.
Kapoor
So Dr. Kapoor summed it up very well, “Although the PTAC did not recommend the proposal, they acknowledged that it met the criteria for adoption as an APM, that it would improve patient care, that it would save program dollars, and it would reduce disparity in access to services. Fundamentally, their rationale was not on the quality of the proposal, but rather one of philosophy. The PTAC’s grounds was that it was inappropriate for specialists to benefit from a total cost of care model where payments were aligned with quality outcomes. All of us, as well as Congress and CMS disagree with that view.” Again, basically this proposal did exactly what Congress said we should do when they passed MACRA.
Conclusion
So, in conclusion, the triple aim set forth in MACRA was met by our proposal. The LUGPA APM qualified as an advanced APM, which going back to Dr. Shore’s talk the other day, is the highest tier of APMs and allows the practices to benefit the most, and the LUGPA APM, as we know from nationwide surveys, appeals to many urologists both in large and small practices, independent or hospital owned, and in practices with or without integrated services. It’s a substantial value for CMS, and as I pointed out, a substantial savings for the country.
Appendix Slides
The rest of the story is that we’re not done yet. We’re still in process. We’re fine-tuning the proposal, and we’ll take it back to CMS later this year. So thank you very much for your attention.
Practice Increases AS from 23% to 33%
Comment from Dr. Raoul S. Concepcion:
I congratulate you on really distilling this down, and I just want the audience to be aware that this is a very long, arduous, and expensive project. Submitting these APMs requires a lot of man hours and a lot of dollars, and I think what is really egregious about the PTAC recommendations and their statements is that the modeling that was used with this care fee of $75 is exactly how the OCM was modeled, which is $160 a month, and PTAC makes the ridiculous statement why should urologists be rewarded for doing the right thing, acknowledging that active surveillance is appropriate therapy, but that is exactly what they are doing to medical oncologists.
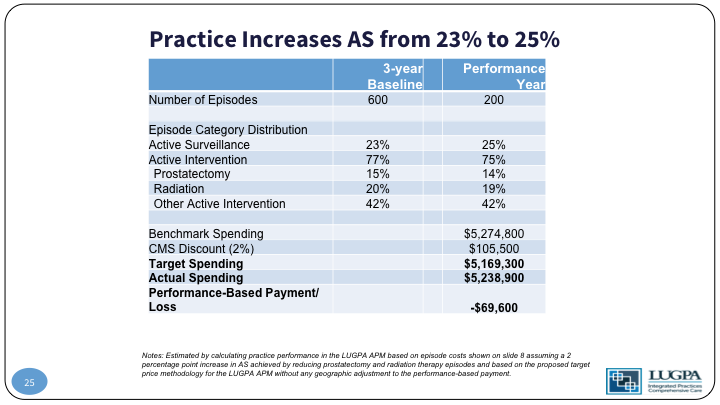
Practice Increases AS from 23% to 25%
Comment from Dr. Thomas Keane:
Urologists, I think, traditionally are known as some of the smarter business people that exist, and LUGPA is perhaps one of the smarter of all of the groups within urology, and I would say these guys are terrified of that proposal, because what it is going to do is set the path for all of the other specialties to follow, and it’s not so much what you guys are putting together, which having listened to it, is completely along the lines of what they wanted. It’s rather terror of what happens if the entire medical community comes onto it, and everybody starts saying, well, we want the same for congestive heart failure, we want the same for stroke patients. Somebody has looked at that and said, oh, this is—we never thought they would be able to do that, and you are. And I think it is a whole new concept for them, and they are probably scared.
ABOUT THE AUTHOR
R. Jonathan Henderson, MD, is a urologist with Arkansas Urology in Little Rock, Arkansas. He obtained a BS in microbiology from Louisiana State University (LSU) in Baton Rouge. After receiving his MD from LSU Medical Center (LSUMC) in Shreveport, he then completed his internship and residency in urology at LSUMC Hospital. During this time, he also authored a number of papers and presentations.
Dr. Henderson spent the next six years in practice in Alabama, where he specialized in laparoscopy and treating disorders of the female bladder. During this time, he served as a representative of Alabama in the Southeastern Section of the American Urology Association. He also served as an assistant clinical professor of urology at the University of Alabama in Tuscaloosa.
Dr. Henderson focuses his practice primarily on robotic surgeries and the treatment of prostate cancer. He is certified by the American Board of Urology. He is a member of the American Urologic Association, the Shreveport Medical Society, the Louisiana State Medical Society, the Society of Laparoscopic Surgeons, and the Alpha Omega Alpha Medical Honor Society. He is past-president of the Large Urology Group Practice Association (LUGPA) and has served on the Board of Directors since 2011.